Hi guys. I'm new on this forum so I apologies for my mistakes.
I found the text below in a book. But does not make sense for me, what the temperature of condensed water (on surface condenser) have to do with amount of work extracted from the motive steam while that temperature is on condenser not on exit from turbine. I mean some energy is extracted by turbine and some by condenser.
So here is the text:
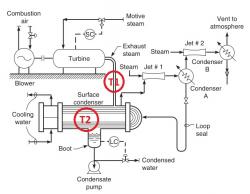
The Second Law of Thermodynamics states: W = (ΔH) (T 2 − T 1 )
where W = amount of work that can be extracted from the motive steam
ΔH = enthalpy of the motive steam minus the enthalpy of the condensed water
T 2 = temperature at which the steam is generated in the boiler
T 1 = temperature at which the steam is condensed in the barometric condenser
The motive-steam supply to a condensing steam turbine is 360°F and 150 psig saturated steam. The turbine is exhausting to a surface condenser. The cooling water to the condenser is 92°F. The turbine is driving a centrifugal compressor. The calculated horsepower produced by the turbine is 10,000 hp.
The colder 62°F well water is to be substituted for the 92°F cooling-tower water. And:
• The steam jets are oversized for the noncondensable flow, which consists of only a very few pounds of air in-leakage.
• The motive-steam flow to the turbine is not known, but won’t change.
• The pressure in the surface condenser is unknown and cannot be measured.
• The cooling-water flow rate is not known, but will not change either. The water will just be colder.
• The efficiency of the turbine and compressor is not known, but is presumed to remain constant.
Calculate the new compression horsepower output from the compressor. Using Eq. W = (ΔH) (T 2 − T 1 ), we note:
• Compression work W is proportional to horsepower.
• ΔH will go up from its prior value a little because the enthalpy of the condensed steam will be lower (because it’s colder).
• As the cooling-water supply is 30°F lower, we will assume that the condensation temperature T 1 in Eq. W = (ΔH) (T 2 − T 1 ) is reduced by 30°F.
• The enthalpy difference between 150-psig saturated steam at 360°F and steam condensed under a good vacuum is roughly 1000 Btu/lb.
• The enthalpy reduction of condensing the steam at a 30°F lower temperature will increase ΔH by 30 Btu/lb.
• ∆H in Eq. W = (ΔH) (T 2 − T 1 ) thus increases by 3 percent.
• T 2 , the temperature of the motive steam, is always 360°F. With 92°F cooling water, we will assume that T 1 is 120°F.
• (T 2 − T 1 ), with 92°F cooling water, is (360°F-120°F) = 240°F.
• T 1 , with 62°F cooling water, is 30°F cooler than T 1 , with 92°F cooling water; that is, the new T 1 is (120°F - 30°F) ? 90°F.
• (T 2 − T 1 ), with 62°F cooling water, is then (360°F ? 90°F) = 270°F.
• With the cooler well water substituted for the warmer cooling-tower water (T 2 - T 1 ) has increased by (270°F-240°F)/240°F=12.5%
Combining the ∆ H effect of 3 percent with the larger (T 2 - T 1 ) effect of 12.5 percent in Eq. (21.1) results in a compression horsepower increase of 16 percent or about 11,600 hp total.
Thanks in advance.
Edited by saneaous, 26 June 2015 - 07:29 AM.

 FB
FB