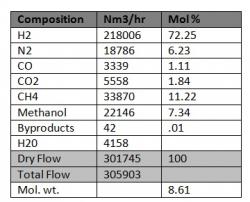
I presume you calculate VLE with Peng-Robinson model since it is the model (from those available in my copy of Prode Properties) which gives similar values (about 0.93 vapor fraction, 0.07 liquid fraction), the model is important as you need to know compositions of vapor and liquid phases in order to evalulate the transport properties, with some other models Prode gives quite different results for H2 72.25 N2 6.23 CO 1.11 CO2 1.84 C1 11.22 CH4O 7.34 C6+ 0.001 so take care (for heat exchangers vapor-liquid equilibria has large influence on enthalpy...) I say this cause I have not investigated if results for VLE are correct (or not)
anyway, with the Peng Robinson model in Prode I get a liquid phase with about 97% methanol, then you can probably assume a pure fluid and use the properties of methanol (available in most sites including NIST)
for vapor phase there are many different methods and procedures which you can adopt, see The Properties of Gases And Liquids for detailed descriptions and many practical examples...
Edited by serra, 04 March 2016 - 11:34 AM.

 FB
FB