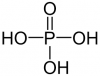
 Dear All,
Dear All,Phosphoric Acid and the derived Phosphate fertilizers play an important role in any economy which has substantial dependence on agriculture. India happens to be one such country where agriculture contributes to approximately 17% of GDP and employs somewhat more than 50% of India's employment.
Although I have never directly worked in the fertilizer sector, I find this area of chemicals quite fascinating from a chemical engineer's perspective. Lately I had been doing some studies on production of wet phosphoric acid and the superphosphates. This led to my developing a material balance spreadsheet for wet phosphoric acid production from commercially mined phosphate rock which I am attaching with this blog entry along with the reference of the literature I have used for developing this spreadsheet. The spreadsheet also includes a process flow scheme for the manufacture of wet phosphoric acid. But before I share the spreadsheet a brief description of the process is essential. Here it goes:
Wet Process for Phosphoric Acid:
Phosphoric acid is produced from fluorapatite, known as phosphate rock, 3Ca3(PO4)2.CaF2, by the addition of concentrated (93%) sulfuric acid in a series of well-stirred reactors. This results in phosphoric acid and calcium sulfate (gypsum) plus other insoluble impurities. Water is added and the gypsum is removed by filtration along with other insoluble materials (e.g. silica). Fluoride, as H2SiF6, is removed at a further stage by evaporation. Although the reaction takes place in stages involving calcium dihydrogenphosphate, the overall reaction can be represented as:
However, there are side reactions; for example with calcium fluoride and calcium carbonate present in the rock:


Fluorosilicilic acid is an important by-product from this.
The crystal structure of the calcium sulfate formed depends on the conditions of the reaction. At 340-350 K, the principal product is dihydrate, CaSO4.2H2O. At 360-380 K, the hemihydrate is produced, CaSO4.1/2H2O.
Calcium sulfate is filtered off and the acid is then concentrated to 56% P2O5 using vacuum distillation.
The product from the 'wet process' acid is impure but can be used, without further purification, for phosphatic fertilizer manufacture such as triple superphophate (TSP), Monoammonium dihydrogenphosphate (MAP) and Diammonium hydrogenphosphate (DAP). Alternatively it can be evaporated further to a concentration of 70% P2O5, a solution called superphosphoric acid which is used directly as a liquid fertilizer.
In my subsequent blog entries I will come out with details of other phosphate fertilizers such as Single Superphosphate (SSP) and Triple Superphosphate (TSP).
The excel spreadsheet for material balance for wet process phosphoric acid (dihydrate) is attached. I would welcome comments on the spreadsheet either to point out improvements or any errors.
Regards,
Ankur.
Attached Files
-
 Phosphoric_Acid_Production.xlsx (110.67KB)
Phosphoric_Acid_Production.xlsx (110.67KB)
downloads: 2043

 FB
FB






Sir, ur work is really inspiring. very few people are good on mass balance calculations. could you also provide calculations for phosphoric acid storage tank calculations. for example how much height and diameter storage tank is required to store 600 mtpd per day of 30% phos acid, so that sludge also settles rapidly