Edited by peterzwart, 12 June 2020 - 05:31 AM.
|
|
Posted 12 June 2020 - 05:25 AM

Edited by peterzwart, 12 June 2020 - 05:31 AM.
Posted 12 June 2020 - 08:44 AM
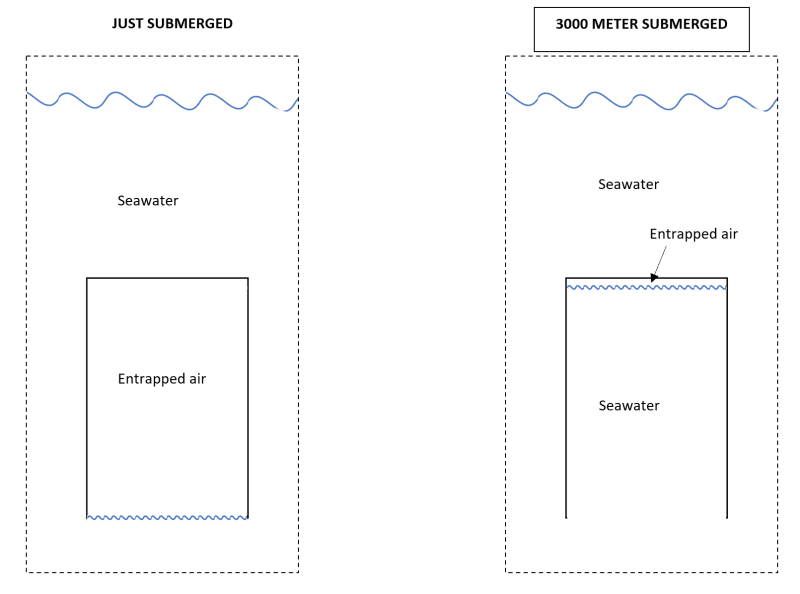
Air can dissolve into the water pretty quickly. I dont really have much rates to show, but I have experience with dissolving air into water at much lower pressures, <10bar.
I would think you can dissolve almost all the air at those kinds of pressures.
I normally refer to saturation curves to see how much air can dissolve in how much water. Unfortunately I don't have one that reaches the pressures you asked for.
Edited by thorium90, 12 June 2020 - 08:47 AM.
Posted 07 July 2020 - 04:56 AM
Thank you for your input Thorium90! Helpful and appreciated.
Peter
Posted 08 July 2020 - 10:04 AM
May be this one is helpful to you. we used this while calculating dissolved oxygen for material selection.
Ammonia Evaporation Rate InquiryStarted by Guest_aslungaard_* , 30 Mar 2024 |
|

|
||
Duplication Of Psv And Flow Rate For Exchanger Tube Rupture ScenarioStarted by Guest_Platonicus_* , 11 Mar 2024 |
|

|
||
How To Calculate Cooling Rate Of CrystallizerStarted by Guest_snehchem_* , 04 Jan 2024 |
|

|
||
Multiple Psvs Relief RateStarted by Guest_fmalik_* , 02 Dec 2023 |
|

|
||
Rate Law For Heterogeneous CatalysisStarted by Guest_DREAMINGNREC_* , 16 Aug 2023 |
|

|