|
|
| More on Ethanol from Cheresources.com: |
| FREE Resources |
| Online Forum: All
Things Ethanol Estimating the New Energy Balance of Corn Ethanol ChE Links: Search for "Ethanol" Students: Ask a Question in our Forums Professionals: Ask a Question in our Forums |
| Ethanol as a Transportation Fuel |
Alcohol has been used as a fuel
for internal combustion engines since their invention. Reports on the use of alcohol
as a motor fuel were published in 1907 and detailed research was conducted in the 1920s
and 1930s. Historically, the level of interest in using alcohol as a motor fuel has
followed cycles of fuel shortages and/or low feed-grain prices.
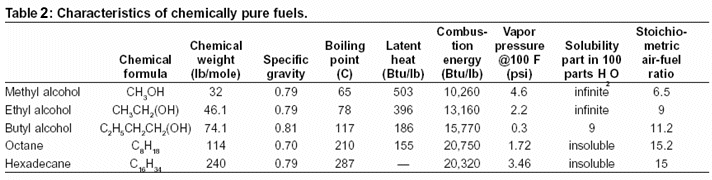
The properties of methyl, ethyl
and butyl alcohol are compared with octane (high quality gasoline) and hexadecane (high
quality diesel fuel) in Table 2. Note that octane and hexadecane (petroleum fuels)
have higher boiling points, lower latent heats and are insoluble in water. The
alcohols become more like petroleum fuels as their chemical weights increase.
Methyl alcohol has the lowest
combustion energy of all the fuels listed. However, it also has the lowest
stoichiometric or "chemically correct" air-fuel ratio. Therefore, an
engine burning methanol would produce the most power. It also is possible to take
advantage of the higher octane ratings of methyl (and ethyl) alcohol and increase the
engine compression ratio. This would increase the efficiency of converting the
potential combustion energy to power. Finally,
Advantages of mixing
alcohol with gasoline are that alcohol tends to increase the octane rating and reduce
carbon monoxide emissions. However, alcohols may corrode certain materials used in
engines.
Table 1: SAMPLE ANHYDROUS
ETHANOL SPECIFICATION |
|
TEST |
SPECIFICATION |
Description/Appearance
|
A clear,
mobile, volatile liquid |
Colour |
Colourless |
Odour |
Characteristic
and spiritous |
Ignition |
Flammable,
burns with a blue smokeless flame |
Solubility |
Must comply
with pharmacopoeia |
Clarity |
The
solution is clear |
Alcoholmetric
test (@ 20 °C) |
Not less
than 99.5 v/v |
Acidity or
Alkalinity |
Complies
with pharmacopoeia |
Reducing
Substances |
Complies
with pharmacopoeia |
Methanol
higher alcohols and esters |
Complies
with pharmacopoeia |
Benzene and
other UV light absorbing substances |
Not more
than 5 ppm |
Heavy Metals |
Complies
with pharmacopoeia |
Residue on
evaporation |
Complies
with pharmacopoeia |
Density @ 20
°C |
788.16 to
791.20 kg/cubic metre |
Aldehydes |
Not more
than 10 ppm |
Blending Alcohol and Gasoline
Mixing alcohol with
gasoline produces gasohol. Advantages of fuel blends are that alcohol tends to
increase the octane rating, which is particularly important in unleaded fuel, and reduce
carbon monoxide (CO) emissions from the engine.
The primary disadvantage
of mixing methanol and ethanol with gasoline is that under certain conditions these
alcohols may separate from the gasoline. An engine adjusted to burn gasoline
efficiently will produce less power from alcohol should it separate from the gasoline.
Separation is caused by the polar nature of the alcohol molecules and their tendency to
absorb water, also a polar substance.
Methanol is the most
likely to separate, butyl alcohol the least likely. The tendency for separation
increases as the temperature decreases, the quantity of water absorbed increases, and the
quality of the gasoline decreases. The effect of using a blend of alcohol and
gasoline
Alcohols as Fuel for Straight-Inline (SI) Engines
Alcohols have high knock resistance, which makes them excellent SI engine fuels. Their latent heat of vaporization is quite high, leading to excellent volumetric efficiency, but can also cause starting problems. Their calorific value is substantially lower than that of gasoline, which would lead to a larger fuel tank volume and also increased jet sizes in carburetors. Alchohols also have low air requirements for complete combustion, however this leads to practically the same mixture enthalpy content. Hence alcohol fueled SI engines can produce the same or even slightly higher power than gasoline.
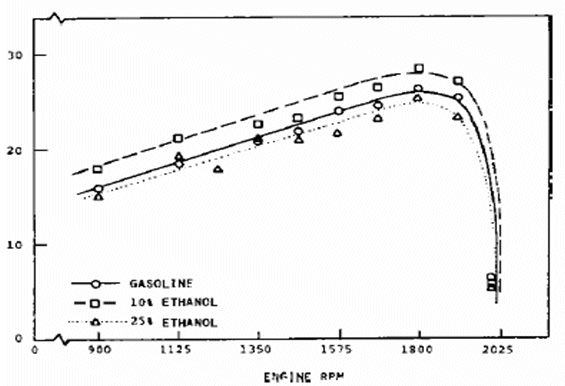 |
| Figure 1: Gasoline engine full throttle power output using ethanol fuel blends |
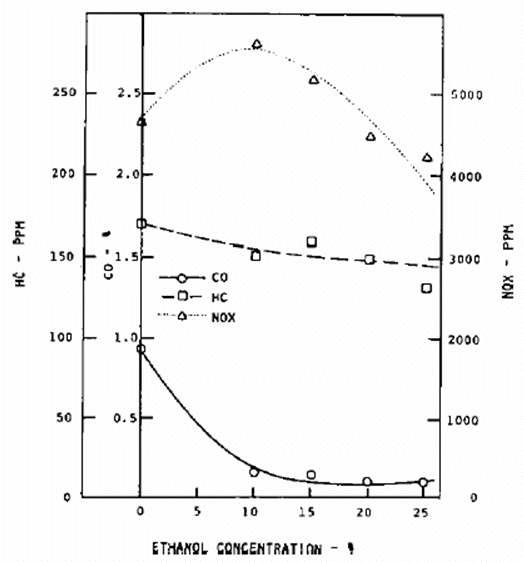 |
| Figure 2: Gasoline engine full throttle exhaust emissions using ethanol fuel blends |
Table 3 compares the
power output and Best Mean Effective Pressure (BMEP) of gasoline and alcohol SI engines
(DB 4.5 litre V8 engine). The higher the octane number of the alcohol allows a
higher compression ratio to be used and the higher
latent heat leads to higher charge induction, enabling the alcohol engine to produce
approximately 10% higher power output.
Table 3: Comparison of Key Engine Factor with Ethanol and
Gasoline
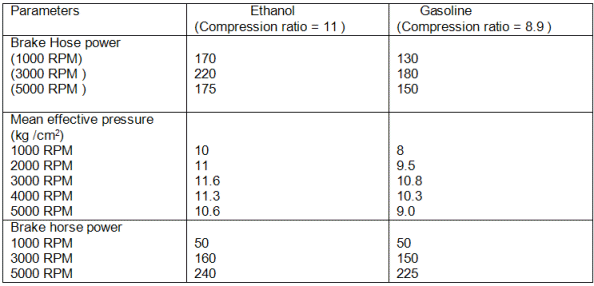
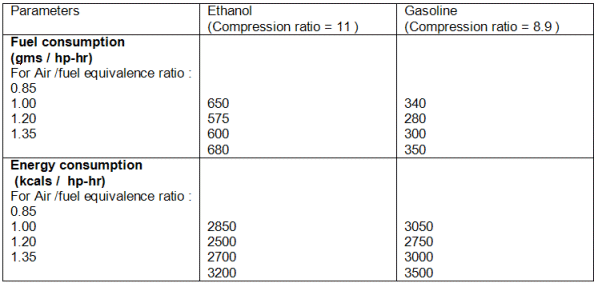
Table 4 shows a
comparison between the specific fuel consumption of the two fuels. On a volumetric
basis, alcohol consumption is much higher due to its lower calorific value. On an
energy basis, however, the superior efficiency of the alcohol engine is reflected in he
significantly lower heat combustion rate.
Table 4: Comparison of Fuel Consumption with Ethanol and Gasoline
Alcohols and Diesel Engines
Alcohol has also been
used in diesel engines. In this case, the alcohol may be blended with diesel fuel to
produce diesohol, or the alcohol may be added to the air intake of the engine. A
system for adding a mixture of ethanol and water to the air intake of a turbocharged
diesel engine is commercially available. The primary function of the system is to
cool the turbocharged air (using the latent heat), and thereby to increase the volumetric
efficiency of the engine and produce more output power. A similar result can be
obtained using an intercooler. Control of the quantity of
Methyl alcohol, because
of its highly polar nature, does not mix with diesel fuel. Ethanol can be mixed with
diesel fuel provided there is little water in the ethanol. A diesel engine normally
will not operate on ethanol nor will ethanol provide lubrication for the fuel injection
system.
Another problem with
adding ethanol to diesel fuel is that the cetane number (ignition characteristic) may
decrease below the level recommended by the engine manufacturer. Butyl alcohol can
be mixed with diesel fuel in virtually any concentration. It does not separate as
water is added or as the temperature is decreased. Further, butyl alcohol does not
significantly change the cetane number of diesel fuel. In blends with diesel fuel,
butyl alcohol tends to reduce the
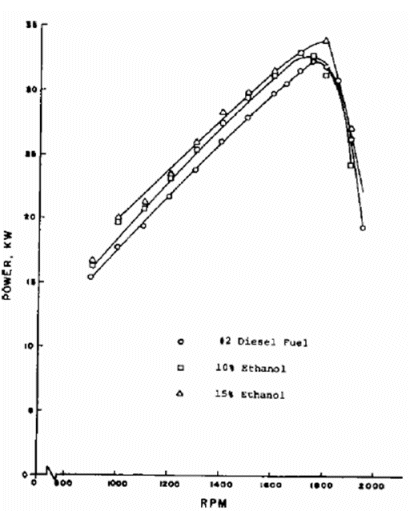 |
| Figure 3: Diesel engine power output using ethanol fuel blends |
A Brief Word on Corrosion
Alcohols may be corrosive
to certain materials used in engines. Generally, methyl alcohol is the most corrosive and
butyl alcohol is least corrosive. Alcohols also can cause injury or physical harm if
not used properly. People who use alcohol in motor fuels should observe warning
labels and follow precautions to avoid problems.
Conclusions
For internal combustion engines, ethanol has the greatest promise as substitutes for petroleum fuels among the alternatives available, at least until the affordable manufacture and effective usage of hydrogen becomes a reality. Alcohols are liquids at normal temperatures, can be manufactured from abundantly available raw materials and have desirable properties of engine fuels. Presently, the major hurdles include their relatively high cost of production and engine adaptation. Storage, transportation and handling without water is another important criterion for the successful use of alcohols as transporation fuels.
By Guest Author Krishnamachari Govindarajan of Petchem Consultants,
Chennai, India
Email: k_govindarajan "at" yahoo.com