Chemical and Process Engineering Resources

Polystyrene is a widely used polymer. After production of the monomer, from one of a few processes, the monomer proceeds to further processing to form polystyrene.
Styrene Monomer Production
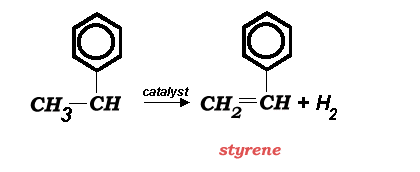
The energy needed for the reaction is supplied by superheated steam (at about 720 °C) that is injected into a vertically mounted fixed bed catalytic reactor with vaporized ethylbenzene. The catalyst is iron oxide based and contains Cr2O3 and a potassium compound (KOH or K2CO3) which act as reaction promoters.Â
After the reaction, the products are cooled rapidly (perhaps even quenched) to prevent polymerization. The product stream (containing styrene, toulene, benzene, and unreacted ethylbenzene) is fractionally condensed after the hydrogen is flashed from the stream. The hydrogen from the reaction is used as fuel to heat the steam (boiler fuel). After adding a polymerization inhibitor (usually a phenol), the styrene is vacuum distilled in a series of four columns (often times packed columns) to reach the required 99.8% purity. The separation is difficult due to the similar boiling points of styrene and ethylbenzene.  Typical capacity per plant ranges from 70,000 to 100,000 metric tonnes per year in each reactor and most plants contain multiple reactors or units.
Polystyrene Production
In 1996, world production capacity for styrene was near 19.2 million metric tonnes per year. Dow Chemical is the world's largest producer with a total capacity of 1.8 million metric tonnes in the USA, Canada, and Europe (1996 figures). The main manufacturing route to styrene is the direct catalytic dehydrogenation of ethylbenzene (above).
The reaction shown above has a heat of reaction of -121 KJ/mol (endothermic). Nearly 65% of all styrene is used to produce polystyrene.
The overall reaction describing the styrene polymerization is:
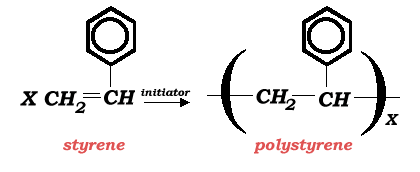
This reaction is carried out in an inert organic solvent environment which provides the reaction medium for this cationic polymerization reaction. The most common solvent used for this reaction is 1,2-dichloroethane (EDC). Other suitable solvents may include carbon tetrachloride, ethyl chloride, methylene dichloride, benzene, toluene, ethylbenzene, or chlorobenzene. The preferred initiator is a mixture of boron trifluoride and water.
The initiator solution is prepared by incorporating 1.5% by weight boron trifluoride gas into the organic solvent (EDC) containing 280 ppm water. This solution is continuously prepared in a holding vessel and will then be injected into the reactor system.
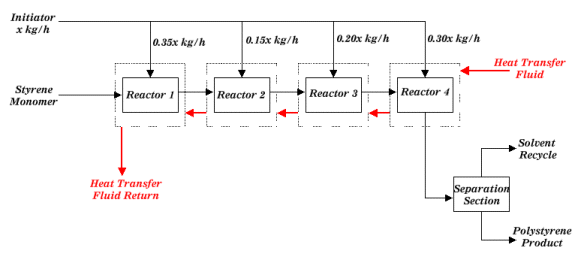 |
| Figure 1: Block Diagrame for Polystyrene Process |
Typical feed to the first reactor would consist of 50 weight percent styrene monomer, 100 ppm water (based on styrene weight), 2000 ppm boron trifluoride (based on styrene weight), with the balance being organic solvent.  The polymerization reaction gives off heat that is carried away from the reactors by jacketing them with a heat transfer fluid. The temperature of the reactants should not vary by more than 15 °C throughout the reactor series.  Temperature control is very important in this reaction because as the reaction temperature increases, the average molecular weight of the polystyrene decreases.  The reaction temperature range is 40-70 °C. Temperature can also be controlled by intermediate shell and tube heat exchangers.
The reaction vessels are typically elongated vessels made of stainless steel. The initiator is introduced as shown below:
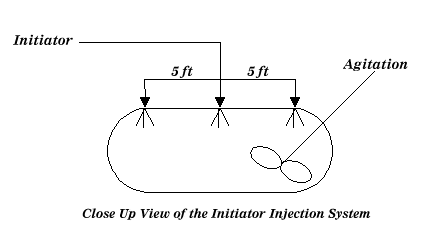 |
| Figure 2: Typical Reactor Overview |
References
- Weissermel, K., Industrial Organic Chemistry, 3rd Edition, VCH, New York, 19972.Â
- US Patent #4161573, assigned to Dow Chemical

 FB
FB

3 Comments
- Solvents are usually feed impurities such as Ethyl benzene, xylenes making it easier to recycle spent monomer.
- each reactor should have its own temperature control loop with an increasing temperature profile as reaction rate decrease drastically with increasing conversion
- before removing residual monomer/ solvents the polymer syrup from the last reactor must be heated to ca. 230-250 C. Residence time in the heater should be minimized to reduce degradation
We manufacture Crystal PS but our color has degraded to light grey because of long residence time of material in heater to remove residual monomers (volatiles). If we lower residence time the volatiles will be higher. What can we do to stabilize the color? Thanks.
Decrease your throughput,