Chemical and Process Engineering Resources
Extractive Distillation: An In-Depth Look
Nov 08 2010 01:40 PM | Chris Haslego
Homogenous Azeotropic Distillation
The most general definition of homogeneous azeotropic distillation is the separation of any single liquid-phase mixture containing one or more azeotropes into the desired pure component or azeotropic products by continuous distillation. Thus, in addition to azeotropic mixtures which require the addition of a miscible separating agent in order to be separated, homogeneous azeotropic distillation also includes self-entrained mixtures that can be separated without the addition of a separating agent.
The first step in the synthesis of a homogeneous azeotropic distillation sequence is to determine the separation objective. Sometimes it is desirable to recover all of the constituents in the mixture as pure components other times it is sufficient to recover only some of the pure components as product. In our example, we would like to recover the cyclohexane product at 90% purity and recycle the separating agent back to the initial separating column for further use.
The second step is to sketch the residue curve map for the mixture to be separated. The residue curve map allows one to determine whether the goal can be reached and if so how to reach it, or the goal needs to be redefined.
Distillation boundaries for continuous distillation are approximated by simple distillation boundaries. It is a good approximation for mixtures with nearly simple distillation boundaries. For a separation to be feasible by distillation, the simple distillation boundary should not be crossed, i.e. the distillate and bottom composition should lie in the same distillation region. A more detail calculation method involving the composition will be discuss in the later section.
In the most common situation, a separating agent is added to separate a minimum boiling binary azeotrope into its two constituent pure components by homogeneous azeotropic distillation. Michael F. D. and Jeffrey P. K. presented seven of the most favorable residue curve maps for this task. Of the seven, the map representing extractive distillation is by far the most common and the most important. Its corresponding residue curve and column sequences are shown in Figure 2 below.
Extractive Distillation
Extractive distillation is defined as distillation in the present of a miscible, high boiling, relatively nonvolatile component, the solvent, that forms no azeotropes with the other components in the mixture. It is widely used in the chemical and petrochemical industries for separating azeotropic, close-boiling, and others low relative volatility mixture.
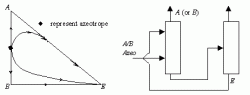 |
| Figure 2: Extractive Distillation with a Heavy Solvent |
Extractive distillation works because the solvent is specially chosen to interact differently with the components of the original mixture, thereby altering their relative volatilities. Because these interactions occur predominantly in the liquid phase , the solvent is continuously added near the top of the extractive distillation column so that an appreciable amount is present in the liquid phase on all of the trays below. The mixture to be separated is added through second feed point further down the column. In the extractive column, the component having the greater volatility, not necessarily the component having the lowest boiling point, is taken overhead as a relatively pure distillate. The other component leaves with the solvent via the column bottoms. The solvent is separated from the remaining components in a second distillation column and then recycled back to the first column.
There are several industrial application for homogeneous azeotropic distillation listed in the Encyclopedia of Separation Technology by Michael F. D., Jeffrey P. K.
Extractive distillations can be divided into three categories, each correspond to the different residue curve maps, the minimum boiling azeotropes, maximum boiling azeotropes and the nonazeotrope mixtures. Since our benzene-cyclohexane mixture to be separated is of the second type of mixture, i.e. the minimum boiling azeotrope, we will focus our attention on column sequencing this type of azeotropic separation method in the following section.
As in azeotropic distillation, design of extractive distillation system will also requires significant preliminary work including:
- Choosing the solvent
- Developing or finding necessary data, such as azeotropic condition or residue curve
- Preliminary screening
- Computer simulation
- Small scale testing
For our example, we will consider the first four steps.

 FB
FB

3 Comments
nice discription. thanks
Thank you for brief explaination.
good