Chemical and Process Engineering Resources
Basics of the Pervaporation System
Figure 3 shows a typical pervaporation system. The feed is allowed to flow along one side of the membrane and a fraction of the feed (permeate) passes through the membrane and leaves in the vapor phase on the opposite side of the membrane.  The "vapor phase" side of the membrane is either kept under a vacuum or it is purged with a stream of inert carrier gas.Â
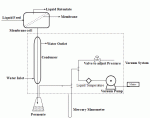 |
| Figure 3: Simplified Pervaporation Process |
Membranes
The membranes used in pervaporation processes are classified according to the nature of the separation being performed. Hydrophilic membranes are used to remove water from organic solutions. These types of membranes are typical made of polymers with glass transition temperatures above room temperatures. Polyvinyl alcohol is an example of a hydrophilic membrane material. Organophilic membranes are used to recover organics from solutions. These membranes are typically made up of elastomer materials (polymers with glass transition temperatures below room temperature).  The flexible nature of these polymers make them ideal for allowing organic to pass through. Examples include nitrile, butadiene rubber, and styrene butadiene rubber.
Factors Affecting Membrane Performance
According to the solution-diffusion model, higher fluxes can be obtained with an increased thermal motion of the polymer chains and the diffusing species. Properties of the polymers that affect diffusion include the "backbone" material, degree of cross-linking, and porosity. Molecular-level interactions between membranes and diffusing species is expressed via a permeability constant used in the Arrhenius relationship:
| Eq. (1) |
Where,
Ep = Activation energy
Po = Permeability constant
RÂ = Gas constant
TÂ = Temperature
Pervaporation Characteristics
Molecular Flux
Molecular flux is the amount of a component permeated per unit area per unit time for a given membrane.
| Eq. (2) |
Where,
Ji = Flux of component "i" (moles/h cm2)
Qi = Moles of component "i" permeated in time "t"
A = Effective membrane surface area (cm2)
Permselectivity
The performance of a given membrane can be expressed in terms of a parameter called permselectivity:
| Eq. (3) |
| Eq. (4) |
Assuming the density of the components in the feed is the same, then:
| Eq. (5) |
Where,
X = Weight fraction
V = Volume fraction
p = Density
Superscripts "p" and "f" denote "permeate" and "feed" respectively while "i" and "j" represent individual components.
Permeability Coefficient
The molecular flux for pervaporation across a membrane can be related to the permeability coefficient by:
| Eq. (6) |
or
| Eq. (7) |
Here ![]() and
and ![]() , therefore
, therefore
| Eq. (8) |
Equation 6 becomes:
| Eq. (9) |
| Eq. (10) |
where:

Industrial Application
Established industrial applications of pervaporation include:
The treatment of wastewater contaminated with organics4
Pollution control applications4
Recovery of valuable organic compounds from process side streams5
Separation of 99.5% pure ethanol-water solutions6
Harvesting of organic substances from fermented broth7
Other products separated or purified by pervaporation include:
| Alcohols | Ketones |
| Methanol | Acetone |
| Ethanol | Butanone |
| Propanol (both isomers) | Methyl isobutyl ketone (MIBK) |
| Butanol (all isomers) | Amines |
| Pentanol (all isomers) | Triethylamine |
| Cyclohexanol | Pyridine |
| Benzyl alcohol | Aniline |
| Aromatics | Aliphatics |
| Benzene | Chlorinated hydrocarbons (various) |
| Toluene | Dichloro methane |
| Phenol | Perchloroethylene |
| Ester | Ethers |
| Methyl acetate | Methyl tert-butyl ether (MTBE) |
| Ethyl acetate | Ethyl tert-butyl ether (ETBE) |
| Butyl acetate | Di-isopropyl ether (DIPE) |
| Organic Acid | Tetrahydro furan (THF) |
| Acetic acid | Dioxane |
Continuing Research on Pervaporation
Pervaporation of Apple Juice
Pervaporation is used to recover any lost juice solution during evaporation. The vapor from the evaporation process is further processed using pervaporation. The recovered, concentrated apple juice can be combined with the product solution to help the apple juice retain it's aromatic and taste qualities.
Pervaporation in the Production of Fuel Ethanol
To establish a continuous fermentation process, the ethanol concentration within the fermentation vessel must be kept at 5% by weight or lower. Pervaporation has been used to maintain the necessary ethanol concentration in the broth. The advantages of using pervaporation in such a system include the ease of processing the clean, nearly pure ethanol extracted from the fermentation vessel and a significantly higher fermentation capacity or the reduction in fermentor size and costs.
Summary
Pervaporation continues to evolve as a feasible separation technology for many different applications. As a proven method of separation as low temperatures and pressure, further application development for food processing is likely. Using pervaporation to clean wastewater streams by removing a variety of organic compounds also holds much promise.
References
1.   Yong Soo Kang, Sang Wook Lee, Un Young Kim and Jyong sup shim, Pervaporation of water – Ethanol mixtures through
      cross – linked and surface modified poly (vinyl alcohol) membrane, J. Member. Sc., Elsevier Science Publishers B.V., Amsterdam, 51, 215, 1990.
2.   K.W. Boddeker and G. Bengston, Pervaporation membranes separation processes, Ed. By R.Y M. Hang. Elsevier, Amsterdam 437 – 460, 1991.
3.   G.H. Koops and C.A. Smolders Pervaporation membrane separation process, Ed. by R.Y.M Haung, Elsevier, Amsterdam 249 –273, 1991.
4.   C. Lipski and P. cote, the use of Pervaporation for removal of organic containment from water, Environmental program, 9, 254 –261, 1990.
5.   J. Kashemekat, J.G. Wiljmans and R.W Baker, Removal of organic solvent containments from industrial effluent streams by       Â
      Pervaporation, Ed. By R. Bakish, Proc. 4th int. Conf. On Pervaporation, process in chemical industry, Bakish materials   Â
      Corporation, Englewood, NJ, 321, 1981.
6.   B.K. Dutta and S.K Sridhar, separation of azeotropic organic liquid mixtures by Pervaporation, AIChE journal, vol.37, No.4, 581– 588, 1991.
7.   M.E.F. Garcia, A.C. Habert, R. Nobrega and L.A. Piers, Use of PDMS and EVA membranes to remove ethanol during     Â
      fermentation, Ed, by R. Bakish Proc. 5th Int. Conf. on Pervaporation process in the chemical industry, Bakish Materials     Â
      corporation, Englewood, NJ, 319 – 330, 1991.
8.   Aptel, P., N. Challard, J. Cuny, and J. Neel, “ Application of the Pervaporation Process to Separate Azeotropic Mixtures,†J.  Â
      Membrane Science., 1, 271 (1976).
9.   Dutta, B.K., D. Randolph, and S.K. Sikdar, “ Separation of Amino Acids Using Composite Ion Exchange Membranes,â€Â  Â
      Biochemical Engineering VI, new York Academy of science, 589, 203,1990

 FB
FB

0 Comments