Chemical and Process Engineering Resources

Distillation is the first step in the processing of crude oil and it takes place in a tall steel tower called a fractionation column. The inside of the column is divided at intervals by horizontal trays. The column is kept very hot at the bottom (the column is insulated) but as different hydrocarbons boil at different temperatures, the temperature gradually reduces towards the top, so that each tray is a little cooler than the one below.
Distillation
The crude needs to be heated up before entering the fractionation column and this is done at first in a series of heat exchangers where heat is taken from other process streams which require cooling before being sent to rundown. Heat is also exchanged against condensing streams from the main column.
As the raw crude oil arriving contains quite a bit of water and salt, it is normally sent for salt removing first, in a piece of equipment called a desalter. Upstream the desalter, the crude is mixed with a water stream, typically about 4 - 6% on feed. Intense mixing takes place over a mixing valve and (optionally) as static mixer. The desalter, a large liquid full vessel, uses an electric field to separate the crude from the water droplets. It operates best at 120 - 150 0C, hence it is conveniently placed somewhere in the middle of the preheat train.
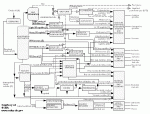 |
| Figure 1: Overview of a Refinery |
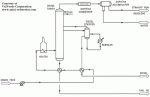 |
| Figure 2: Refinery Distillation Column |
Part of the salts contained in the crude oil, particularly magnesium chloride, are hydrolysable at temperatures above 120 0C. Upon hydrolysis, the chlorides get converted into hydrochloric acid, which will find its way to the distillation column's overhead where it will corrode the overhead condensers. A good performing desalter can remove about 90% of the salt in raw crude.
Downstream the desalter, crude is further heated up with heat exchangers, and starts vaporising, which will increase the system pressure drop. At about 170 -200 0C, the crude will enter a 'pre-flashvessel', operating at about 2 - 5 barg, where the vapours are separated from the remaining liquid. Vapours are directly sent to the fractionation column, and by doing so, the hydraulic load on the remainder of the crude preheat train and furnace is reduced (smaller piping and pumps).
Just upstream the preflash vessel, a small caustic stream is mixed with the crude, in order to neutralise any hydrochloric acid formed by hydrolysis. The sodium chloride formed will leave the fractionation column via the bottom residue stream. The dosing rate of caustic is adjusted based on chloride measurements in the overhead vessel (typically 10 - 20 ppm).
At about 200 - 280 0C the crude enters the furnace where it is heated up further to about 330 -370 0C. The furnace outlet stream is sent directly to the fractionation column. Here, it is separated into a number of fractions, each having a particular boiling range.
At 350 0C, and about 1 barg, most of the fractions in the crude oil vapourise and rise up the column through perforations in the trays, losing heat as they rise. When each fraction reaches the tray where the temperature is just below its own boiling point, it condenses and changes back into liquid phase. A continuous liquid phase is flowing by gravity through 'downcomers' from tray to tray downwards. In this way, the different fractions are gradually separated from each other on the trays of the fractionation column. The heaviest fractions condense on the lower trays and the lighter fractions condense on the trays higher up in the column. At different elevations in the column, with special trays called draw-off trays, fractions can be drawn out on gravity through pipes, for further processing in the refinery.
At top of the column, vapours leave through a pipe and are routed to an overhead condenser, typically cooled by air fin-fans. At the outlet of the overhead condensers, at temperature about 40 0C, a mixture of gas, and liquid naphtha exists, which is falling into an overhead accumulator. Gases are routed to a compressor for further recovery of LPG (C3/C4), while the liquids (gasoline) are pumped to a hydrotreater unit for sulfur removal.
A fractionation column needs a flow of condensing liquid downwards in order to provide a driving force for separation between light and heavy fractions. At the top of the column this liquid flow is provided by pumping a stream back from the overhead accumulator into the column. Unfortunately, a lot of the heat provided by the furnace to vaporise hydrocarbons is lost against ambient air in the overhead fin-fan coolers. A clever way of preventing this heat lost of condensing hydrocarbons is done via the circulating refluxes of the column. In a circulating reflux, a hot side draw-off from the column is pumped through a series of heat exchangers (against crude for instance), where the stream is cooled down. The cool stream is sent back into the column at a higher elevation, where it is been brought in contact with hotter rising vapours. This provides an internal condensing mechanism inside the column, in a similar way as the top reflux does which is sent back from the overhead accumulator. The main objective of a circulating reflux therefore is to recover heat from condensing vapours. A fractionating column will have several (typically three) of such refluxes, each providing sufficient liquid flow down the corresponding section of the column. An additional advantage of having circulating refluxes is that it will reduce the vapour load when going upwards in the column. This provided the opportunity to have a smaller column diameter for top sections of the tower. Such a reduction in diameter is called a 'swage'.
The lightest side draw-off from the fractionating column is a fraction called kerosene, boiling in the range 160 - 280 0C, which falls down through a pipe into a smaller column called 'side-stripper'. The purpose of the side stripper is to remove very light hydrocarbons by using steam injection or an external heater called 'reboiler'. The stripping steam rate, or reboiled duty is controlled such as to meet the flashpoint specification of the product. Similarly to the atmospheric column, the side stripper has fractionating trays for providing contact between vapour and liquid. The vapours produced from the top of the side stripper are routed back via pipe into the fractionating column.
The second and third (optional) side draw-offs from the main fractionating column are gasoil fractions, boiling in the range 200 - 400 0C, which are ultimately used for blending the final diesel product. Similar as with the kerosene product, the gasoil fractions (light and heavy gasoil) are first sent to a side stripper before being routed to further treating units.
At the bottom of the fractionation column a heavy, brown/black coloured fraction called residue is drawn off. In order to strip all light hydrocarbons from this fraction properly, the bottom section of the column is equipped with a set of stripping trays, which are operated by injecting some stripping steam (1 - 3% on bottom product) into the bottom of the column.

 FB
FB

7 Comments
Thank u Sir !
Great article to understand the functions of whole refinery, !!