Chemical and Process Engineering Resources
The Latent Image and Image Development
The silver halide process is by far the most important of all of the radiation-sensitive photographic systems in use today. The principal reason for this superiority is the high sensitivity of the system - the amount of radiant energy required to produce a useful image – and the extreme flexibility of the system in terms of adjusting sensitivity, contrast, tonal range and other such aspects of the product. The impact of a single photon on a silver halide grain, for example, produces a nucleus of at least four silver atoms, and that effect can be amplified as much as a billion times by the action of a properly chosen reducing agent or "developer."
The silver halides employed are silver bromide, silver chloride and silver iodide. The first two may be used separately or combined, depending on the sensitivity and tonal qualities desired in the product. Silver iodide is always combined with silver bromide or silver chloride.
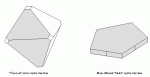 |
| Figure 2: A Schematic Representation of Cubic and "Tablet" Silver Halide Grains |
As already noted, the silver halides used in photography are dispersions of microscopic crystals in a colloidal binder that is usually bone gelatin. Although such dispersions are referred to as emulsions or photographic emulsions, they are really dispersions.
When an exposed film is placed in a developer solution, the grains that contain silver nuclei are reduced much faster than those that do not. The more nuclei present in a given grain (i.e., the greater the exposure of that grain), the faster the reaction with developer and the darker the image at that site in the film. Factors such as temperature, concentration of the developer, pH, and the total number of nuclei in each grain determine the extent of development and the intensity of free silver (blackness) deposited in the film emulsion in a given time.
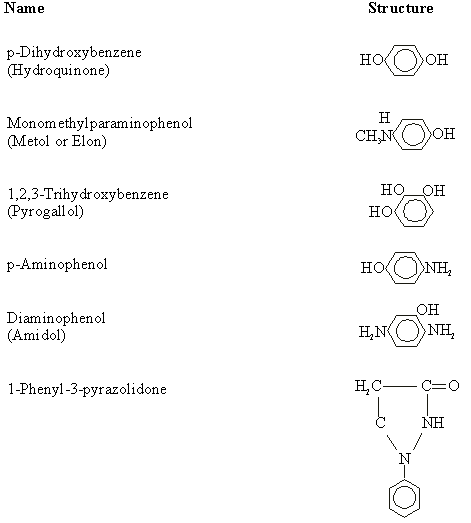
Not only must the developer be capable of reducing silver ions to free silver, but it must be selective enough not to reduce the unexposed grains, a process known as "fogging," within the time frame of the development process. Some substances that are commonly used as silver halide developers are listed in Table 1.
The developer is oxidized in the process. If not properly protected it can also be oxidized by air, a process that, if not prevented, will result in the loss of developer activity. To help prevent such effects, commercial developer solutions commonly contain preservatives such as sodium sulfite.
Gelatin - The Film Matrix
Gelatin is a protein extracted from animal hides, bone, and sinew that belongs to the class of substances known as hydrophilic ("water loving") polymers, which also includes other proteins, gums, starches, and a wide variety of synthetic polymers. Photographic grade gelatin is usually produced by an alkaline extraction process using bovine bones, although some acidic processes have been developed. Some special gelatins are also used that are derived from pigskins. The origin and quality of the raw materials used in the gelatin process, the conditions employed (pH, temperature, time, etc.), and the presence or absence of certain possible contaminants (e.g., mercury or other heavy metals, lipids, sulfur compounds, etc.) are of vital importance to the production of material suitable for use in modern photographic systems. In fact, photographic gelatin is much purer that that used in food applications.
 |
Gelatin solutions have the useful property of behaving as a liquid when warm and setting to a relatively hard gel when cool. When coated on a substrate and dried it is flexible and reasonably resistant to physical damage, but it readily absorbs water (as in the development process). Generally, the coated gelatin dispersion will contain some material such as formaldehyde as a "hardener" that serves to produce a limited number of cross links among protein chains that improve the physical characteristics of the coated material. The gelatin swells rapidly, absorbing water and dissolved development chemicals, but it does not dissolve or disintegrate at normal temperatures. These properties are essential in the preparation of a photographic emulsion, in coating it on film base or paper, and in the processes of development, fixation and washing.
Gelatin is more than a means of holding the sensitive silver halide salts in place on a on a film base, however. It is an integral part of the process and can significantly affect the properties of the final photographic product. Controlling the characteristics of the gelatin is absolutely essential to the photographic emulsion preparation, the process of coating the emulsion on the film base, the adhesion of the coated material to the film base, the wetting characteristics of the coated material in the development process, the physical characteristics of the dried developed product, and the long-term stability of the developed image. In a way, one could safely say that the gelatin is almost as important as silver to the overall photographic system.
In the preparation of a photographic emulsion the gelatin also acts as an anti-coagulant or stabilizing colloid. The silver halide formed in fluid gelatin does not precipitate out of solution but remains uniformly distributed throughout the preparation, ripening (see below), and coating processes. The gelatin is an important factor in determining the dispersity or range of grain sizes of the silver halide. By suitable regulation of the concentration of the gel, the temperature, and the rate of addition of the components, the grain size distribution can be controlled to meet specific requirements.
In the silver halide dispersion, gelatin molecules adsorb at the surface of the silver halide grain, surrounding the grain and forming a barrier that stabilizes the dispersion. The adsorbed layer also, in all likelihood, affects the radiation sensitivity of the grain and makes reduction by developers, more controllable. This is important in the development process and makes it possible to obtain desired results from a given system based on easily controlled parameters such as developer chemistry, development time, temperature, etc.

 FB
FB

0 Comments