Chemical and Process Engineering Resources
Color Sensitizing
The radiation sensitivity of silver halides ends for all practical purposes at about 525 mm . In Figure 3 Curve A illustrates the spectral sensitivity of a typical silver bromo-iodide emulsion and B illustrates the average human visual response curve. As the curves show, the maximum response of the eye is in the yellow-green near 550 mm which lies beyond the sensitivity range of the emulsion, which is much more sensitive to the violet and blue than the eye.
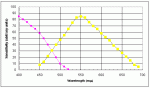 |
| Figure 3: Curves Approximating the Light Sensitivity of Typical Silver Halide Crystals (blue) and the Human Eye (yellow) |
The sensitivity of the silver halides may be extended to radiation of longer wavelengths by the addition of dyes or "color sensitizers." Although referred to as dyes, color sensitizers are not ordinary dyes in that they are not used to color cloth or other materials.
Emulsion sensitizing results from the absorption of radiant energy by the dye at a wavelength that would not affect the silver halide, and the transfer of that "exposure" energy to the silver halide to form a latent image and make the affected grain developable. If a dye is a sensitizer, then its action depends upon the absorption characteristics of the silver halide-adsorbed color sensitizer complex, which may be quite different from the absorption of the dye itself. Since the sensitivity of such dyes varies greatly, it is often necessary to use a combination of materials to obtain a specific result. Some combinations, however, do not work well together, so that the system balance must be carefully studied before the final emulsion composition is determined. There are substances that may or may not be sensitizers themselves but greatly increase the sensitizing action of other dyes. These are known as super-sensitizers and are of considerable importance in facilitating the use of conventional sensitizing dyes. Whatever the dye used, the quantity required is always quite small.
Development
The rate of development of the individual grains in an emulsion is affected by so many factors, such as the rate of diffusion of the solution through the gelatin matrix, the adsorption of the developing agent, the solution of the silver halide, oxidation products of the developing agent and the accumulation of restraining by-products, that exact analysis of it is difficult. The time of appearance of a visible image is, within limits, a reliable indication of the rate of development. It varies with different emulsions and is quite different with different developing agents, but the variation with temperature, dilution and pH is almost directly related to the variation in the rate of development.
The rate of development, as determined from the change in the optical density of the developed image, is complicated by the fact that density increases in two different ways: (1) by the increase in the amount of silver as the grains develop and (2) by an increase in the number of grains in the process of development. Density grows rapidly at first and then slows down until development is complete and no further growth in density takes place. Prolonged development would, of course, increase overall density through the development of unexposed grains (fog).
Halting Development - The Stop Bath
Once the exposed image has been developed to the desired degree, it is necessary to halt the chemical process quickly to prevent over development and the production of fog. The solution used to that end is referred to as the "stop" bath. Since developers function a relatively high pH’s, the typical stop bath is simply a solution of acetic or some other weak acid. The action of the acid is so rapid it usually requires only seconds for the process to be effectively halted.
The Fixing Process
Once the developed image is obtained, a large amount of unexposed and undeveloped silver halide remains in the emulsion. If that silver halide is not removed before the image is exposed to radiation capable of producing a latent image, the image will continue to darken. The process of removing the residual silver halide from the image is called "fixing."
The silver halides are only slightly soluble in water; therefore, to remove the material remaining after development it is necessary to convert it to soluble complexes which can he removed by washing. Sodium thiosulfate, commonly termed "hypo," has been used for this purpose since 1839.
The reactions in fixing can be written as follows:
AgBr + S2O3-2 --> AgS2O3- + Br- (adsorption complex)
which is followed by
AgS2O3- + S2O3-2 --> Ag(S203)2-3 (desorbed)
and by
Ag(S203)2-3 <--> AgS2O3- + S2O3 -2; AgS2O3 <--> Ag+ + S2O3-2
Within limits, the rate of fixation is indicated by the clearing time, i.e., the time required to remove all visible traces of silver halide from the image. This time depends on the concentration of thiosulfate, the temperature, the agitation of the solution, but more particularly on the emulsion and the extent to which the fixing bath has been used. Fine-grain emulsions fix in less time than those of larger grains, and paper emulsions of silver chloride fix faster than bromo-iodide negative emulsions. Thickly coated films, other things being equal, fix more slowly than those with a thin emulsion coating. The fixing time increases appreciably as the solution becomes depleted. With continued use the halide-ion concentration rises in proportion to the amount of silver halide dissolved. When the product of the silver-ion and the halide-ion activities reaches the solubility product of the least soluble silver halide present, the solution will dissolve no more of that silver halide and fixation will necessarily be incomplete
It is usually desirable to harden the gelatin after development, and while this may be accomplished by a hardening stop bath prior to fixing, the usual practice is to combine hardening with fixing. The conventional fixing and hardening bath contains in addition to the fixing agent:
1. An organic acid, usually acetic, to provide the necessary acidity to stop development and create the proper pH for effective hardening.
2. Sodium sulfite, which prevents the decomposition of the thiosulfate by the acid and forms colorless oxidation products of the developer thus preventing staining.
3. Alum as a hardening agent.
The hardening produced by alum is due to the reaction of the aluminum ions, Al+3, and the carboxyl groups of the gelatin with the formation of cross-linkages between chain molecules. The degree of hardening, other things such as temperature, alkalinity of the film when placed in the fixing bath, etc., being equal, depends on the pH of the solution which in turn depends on the relative proportions of acid, sulfite and alum.
Since the addition of developer tends to increase the pH of the fixing bath, the solution should be buffered against an increase in pH. For this reason weak organic acids, such as acetic acid, are used in preference to a stronger acid, such as sulfuric. The addition of boric acid increases the useful hardening life of potassium alum baths and reduces the tendency of the bath to form a sludge.
Reference and Further Reading
- Mees, C.E.K, and James, T.H., The Theory of the Photographic Process, 3rd ed., The Macmillan Co., New York, 1966.
- Neblette, C.B., Fundamentals of Photography, Van Norstrand Reinhold Co., Princeton, N.J., 1970.
- Croome, R.J., Photographic Gelatin, Focal Press, New York, 1965.

 FB
FB

0 Comments