Chemical and Process Engineering Resources

Sources of Biomass for Biosorption
Sources of biomass include:
- Seaweeds
- Microorganisms (bacteria, fungi, yeast, molds)
- Activated sludge
- Fermentation waste
- Other specially propagated biomasses.
Biosorbents must be hard enough to withstand the application pressures, porous and/or "transparent" to metal ion sorbate species, and have high and fast sorption uptake even after repeated regeneration cycles17.
Granulation of biomass materials into suitable cost-effective biosorbents is a crucial step for the successful application of biosorption processes.
The objectives of granulation are to:
- Establish the behavior of native biomass in a packed-bed reactor
- Establish the effectiveness of biomass granulation and reinforcement
- Determine the effect of size reduction on sorption capacity
- Determine the feasibility of biomass processing.
Conventional granulation technologies are rather advanced, and their adaptation will likely yield desirable biosorbent granules5. Because of the wide variety of biomass types, extensive experimentation will undoubtedly be required.
The need to transport raw biomass may also present some logistical problems. Microbial biomass has a high water content and is prone to decay, so drying may be required if it cannot be processed and/or granulated directly on location in the wet state.
Equilibrium Modeling
Biosorption has been studied as simplified sorption systems, usually containing one heavy metal. This is an appropriate simplification for effective experimentation.
Table 2 summarizes some of the simple sorption isotherm models that are most frequently applied. A particular model may not apply to a particular situation, and in some cases more than one model may explain the biosorption mechanism. There is no critical reason to use a more-complex model if a two-parameter model (such as the Langmuir and Freundlich isotherm models) can fit the data reasonably well.
| Isotherm | Equation | Advantages | Disadvantages |
| Langmuir | 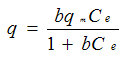 | Interpretableparameters | Not structured; Monolayer sorption |
| Freundlich | Simpleexpression | Not structured | |
| Combination of Langmuir and Freundlich | 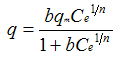 | Combination of the above two | Unnecessarily complicated |
| Radke and Prausnitz | 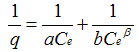 | Simple expression | Empiral; Requires three parameters |
| Redlich Peterson | 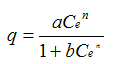 | Approaches Freundlich at higher concentrations | No significant advantages |
| Braunauer, Emmer, and Teller (BET) | 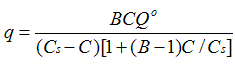 | Multilayer adsorption | ------ |
| Dubinin-Radushkevich | 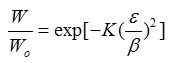 | Temperature dependent | Behavior is not limited in the Henry''s Law regime |
As a matter of practicality, multi-metal biosorption models such as those in Table 3 must be used judiciously.
| Isotherm | Equation | Advantages | Disadvantages |
| Langmuir (Multi-component) | 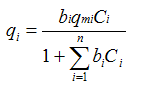 | Constants have physical meaning; | Not structured; doesn''t reflect the mechanism well |
| Combination (Langmuir and Freundlich) | 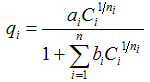 | Combination of Langmuir and Freundlich | Unnecessarily complicated |
where:
Ce is the equilibrium solute concentration in the fluid
K,n are the Freundlich isotherm constants
ai,bi are the Langmuir isotherm parameters
e is the column bed porosity; Polanyi''s adsorption potential
qm is the Langmuir maximum metal uptake in mg/g
Wo,Wm are the initial and final volumes, respectively, in L
K(with various subscripts) are the intrinsic equilibrium constants
Q is the meta uptake in mg/g
b is the Polanyi scaling factor in the Polanyi models.
The sorption uptake, q, can be expressed in different units depending on the purpose of the exercise:
- For practical and engineering process evaluation purposes eventually concerned with process mass balances, it is customary to use weight per (dry) weight (e.g., mg of metal sorbed per gram of the (dry) sorbent material).
- Ultimately, mainly because of reactor volume considerations (e.g., a packed-bed column), the uptake may also be expressed on a per volume basis (e.g., mg/L). However, the porosity may complicate the quantitative comparison of biosorption performance.
- Only when working on the stoichiometry of the process and when studying the functional groups and metal-binding mechanisms might it be useful to express q on a molar or charge equivalent basis - again, per unit weight or volume of the sorbent (e.g., mmol/g or mequiv/g).
It is relatively easy to convert among these units; the only problem may arise with the sorbent weight-volume conversions. For scientific interpretations, the sorbent material dry-weight basis is thus preferred.
The use of "wet biomass weight" should be discouraged, unless the wet-weight-to-dry-weight conversion is well specified. Different biomass types are likely to retain different moisture contents, intracellular as well as that trapped in the interstitial space between the cells or tissue particles (e.g., seaweed particles). Different types of biomass obviously compact in a different ways. When centrifuging biomass, the g-force and time need to be specified, and even then it is difficult to make any comparisons. All this makes the "wet biomass weight" citation very approximate at best and generally undesirable.

 FB
FB

0 Comments