Chemical and Process Engineering Resources

Crystallization refers to the formation of solid crystals from a homogeneous solution. It is essentially a solid-liquid separation technique and a very important one at that.
Crystals are grown in many shapes, which are dependent upon downstream processing or final product requirements.Â
Understanding the Basics of Crystallization
As with any separation method, equilibrium plays an important role. Below is a general solubility curve for a solid that forms hydrate (a compound that has one or more water molecules attached) as it cools.
In Figure 1, X may be any solid that can form hydrates such as Na2S2O3. The number of hydrate molecules shown in Figure 1 is strictly arbitrary and will vary for each substance.
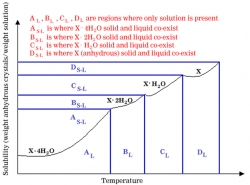 |
| Figure 1: Example of a Solubility Curve |
 So how do you grow crystals? Let's consider an example that is fairly easy to envision. Take a pot of boiling water and add table salt while stirring to make a water-salt solution. Continue adding salt until no more salt will dissolve in the solution (this is a saturated solution). Now add one final teaspoon of salt. The salt that will not dissolve will help the first step in crystallization begin. This first step is called "nucleation" or primary nucleation. The salt resting at the bottom of the pot will provide a site for nucleation to occur.
On an industrial scale, a large supersaturation driving force is necessary to initiate primary nucleation. The initiation of primary nucleation via this driving force is not fully understood which makes it difficult to model (experiments are the best guide). Usually, the instantaneous formation of many nuclei can be observed "crashing out" of the solution. You can think of the supersaturation driving force as being created by a combination of high solute concentration and rapid cooling. In the salt example, cooling will be gradual so we need to provide a "seed" for the crystals to grow on.  In continuous crystallization, once primary nucleation has begun, the crystal size distribution begins to take shape. Think about our salty water, as you look at Figure 2 describing the progression of crystallization.
Examples of Crystallization
|
 The second chief mechanism in crystallization is called secondary nucleation. In this phase of crystallization, crystal growth is initiated with contact. The contact can be between the solution and other crystals, a mixer blade, a pipe, a vessel wall, etc. This phase of crystallization occurs at lower supersaturation (than primary nucleation) where crystal growth is optimal.  Â
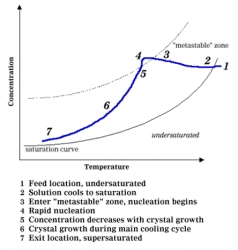 |
| Figure 2: Progression of Crystallization |
Again, no complete theory is available to model secondary nucleation and it's behavior can only be anticipated by experimentation. Mathematic relationships do exist to correlate experimental data. However, correlating experimental data to model crystallization is time consuming and often considered extreme for batch operations, but can easily be justified for continuous processes where larger capital expenditures are necessary. For batch operations, only preliminary data measurements are truly necessary.
We've discussed how crystallization occurs once supersaturation is reached, but how do we reach supersaturation? We have already covered one such method in our salt crystallization example. Since the solubility of salt in water decreases with decreasing temperature, as the solution cools, its saturation increases until it reaches supersaturation and crystallization begins (Figure 3). Cooling is one of the four most common methods of achieving supersaturation. It should be noted that cooling will only help reach supersaturation in systems where solubility and temperature are directly related. Although this is nearly always the case, there are exceptions. In Figure 3, you'll note that Ce2(SO4)3 actually becomes less soluble in water at higher temperatures.Â
| Secondary nucleation requires "seeds" or existing crystals to perpetuate crystal growth. In our salt example, we bypassed primary nucleation by "seeding" the solution with a final teaspoon of salt.  Secondary nucleation can be thought of as the workhorse of crystallization. |
Â
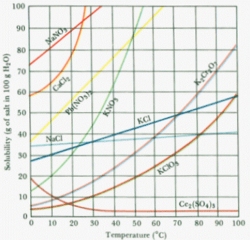 |
| Figure 3: Solubilities of Several Solids |
The four most common methods of reaching supersaturation in industrial processes are:
- Cooling (with some exceptions)
- Solvent Evaporation
- Drowning
- Chemical Reaction
In an industrial setting, the solute-solvent mixture is commonly referred to as the "mother liquor".
Drowning describes the addition of a nonsolvent to the solution which decreases the solubility of the solid. A chemical reaction can be used to alter the dissolved solid to decrease its solubility in the solvent, thus working toward supersaturation. Each method of achieving supersaturation has its own benefits. For cooling and evaporative crystallization, supersaturation can be generated near a heat transfer surface and usually at moderate rates. Drowning or reactive crystallization allows for localized, rapid crystallization where the mixing mechanism can exert significant influence on the product characteristics.

 FB
FB


0 Comments