Below is a try to clarify the matter, despite my lack of relevant experience.
1. W. L. Nelson in 'Petroleum Refinery Engineering' (McGraw-Hill, 1958), Table 4-6, presents salt content of several crudes, with average value varying from 1 to 261 lb salt per 1000 bbl. Thus reported 0.018 lb/1000 bbl must be incorrect.
2. Assuming a crude oil sg=0.9, and that measured Cl is 10 ppm w/w, NaCl content can be calculated as follows:
NaCl content =10*1.65=16.5 ppm w/w. Volume of 1000 bbl=159 m3. Mass of 1000 bbl crude=159*0.9*1000=143100 kg =315476 lb. NaCl content in 1000 bbl crude=315476*16.5E-6 = 5.2 lb. Found value of 5.2 PTB is close to 5.78 PTB, corresponding to crude oil sg=1.0 (exceptionally heavy crude, but possible; see Nelson, ibid, appendices 1 & 2).
3. Consequently the right value is 5.78 PTB.
4. An interpretation of the formula in Manning & Thompson book:
4.1 First two terms represent volume of Sediment & Water (S&W) per 1000 bbl of crude, although denominator (100-%S&W) is not clear to me. Volume of sediment is considered negligible compared to water volume.
4.2 (350*SGbrine) represents brine density in lb/bbl.
4.3 So (ppmw) refers to NaCl content in brine(w/w), not in total crude. This could explain the difference reported.
kkala. I did mentioned the difference of salt content value by using the actual value of crude oil density = 0.819 in our refinery, however the Chemist in my workplace told me that as the chloride is extracted from crude oil using the water during the titration method, then SG = 1 must be used.
I know this is not an accurate practice as we are converting 1000 bbl to mass and we need to convert it using crude oil SG.

This is his response :
[The chloride measurement for salt content was carried out in aqueous layer based on our method. Water is used to extract the salt from the crude and that was a reason why we didn’t border about the crude density. Unless you are using ASTM D3230 for salt measurement definitely you ought to consider density variation for each crude in the calculation.
Do you have any idea?
Edited by sp3d2, 20 July 2011 - 10:02 PM.
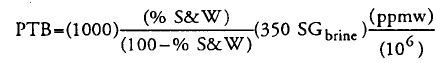

 FB
FB











