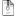Stream "mixture" has a temperature of 59.85C and 3937kPa. Stream "out" you have set as 1042kPa. One side of your process has a valve that expands and therefore cools down the fluid to a lower temperature. The other side of your process has an expander unit that also expands and therefore cools down the fluid to a lower temperature. So how can you put a unit op to adjust the split ratio between the two streams so that you get back the same 59.85C when both sides will get cooled down below 59.85C and will never be able to achieve it? Your simple process makes no meaning, perhaps you can explain what is it you are trying to model?
@thorium:
You are probably right and there's a blunder.
But I just wanted to get your opinion on an alternative scenario I was thinking. Tell me if it is wrong or possible.
Say @sacarove's fluid has a lot of H2 in it (I'm not sure, what is the composition?). H2 will still cool on pressure reduction through an isentropic expander (t), but since H2 has an inversion temp. of ~ -70 C it would heat up when throttled through a valve (v) at his Temperature of ~50 C, right?
In which case, there ought to be a certain split that may indeed be possible that would drop pressures without a net cooling? The strategy seems in theory not impossible?
Thoughts?
PS. I just noticed that his first sketch shows H2O + Oil + Gas so my assumption of a H2 rich stream seems bad. Which makes the rest of my argument academic. Can't think of any other reasonable gas combinations that have an inversion temperature below ambient.
@sacrove:
Might you be overconstraining the problem? Perhaps you'll have to let the downstream pressure also float if the downstream T = upstream T is your hard constraint? Just a thought.
Edited by curious_cat, 07 October 2013 - 06:14 AM.

 FB
FB