|
|
Hydrocarbon Mixture Dewpoint
Started by siddiqsh, Apr 13 2009 11:55 PM
9 replies to this topic
Share this topic:
#1

Posted 13 April 2009 - 11:55 PM
I need to calculate dewpoint of hydrocarbon mixture having ethane 5%, ethylene 30%, hexane 3% and rest nitrogen 62%(mole fraction). pressure 24 kg/cm²g, temperature 85°C. please give me steps for calculating dewpoint
#2

Posted 14 April 2009 - 12:35 AM
Dew Point is 49.18 Deg C; i have used a simulator for this calculation.
#3

Posted 14 April 2009 - 08:57 AM
Siddiqsh,
Check out this on-line calculator:
http://www2.questcon...rmo/dewbub.html
Seems to be fairly good.
Regards,
Ankur.
#4

Posted 14 April 2009 - 10:06 PM
Hi Siddiqsh,
You may like to try it by hand using the classic DePriester Charts. It may be approximate, but I really recommend that you try it by hand.
I have attached two diagrams for your reference.
Have fun!
 DePriester_Chart_High_T_SI.pdf 1015.72KB
152 downloads
DePriester_Chart_High_T_SI.pdf 1015.72KB
152 downloads
 DePriester_Chart_low_T_SI_units.pdf 592.19KB
96 downloads
DePriester_Chart_low_T_SI_units.pdf 592.19KB
96 downloads
You may like to try it by hand using the classic DePriester Charts. It may be approximate, but I really recommend that you try it by hand.
I have attached two diagrams for your reference.
Have fun!
 DePriester_Chart_High_T_SI.pdf 1015.72KB
152 downloads
DePriester_Chart_High_T_SI.pdf 1015.72KB
152 downloads DePriester_Chart_low_T_SI_units.pdf 592.19KB
96 downloads
DePriester_Chart_low_T_SI_units.pdf 592.19KB
96 downloads
#5

Posted 19 April 2009 - 05:51 AM
thanks for your time.
I am looking for some method for calculating dewpoint using some equations.
#6

Posted 19 April 2009 - 08:27 AM
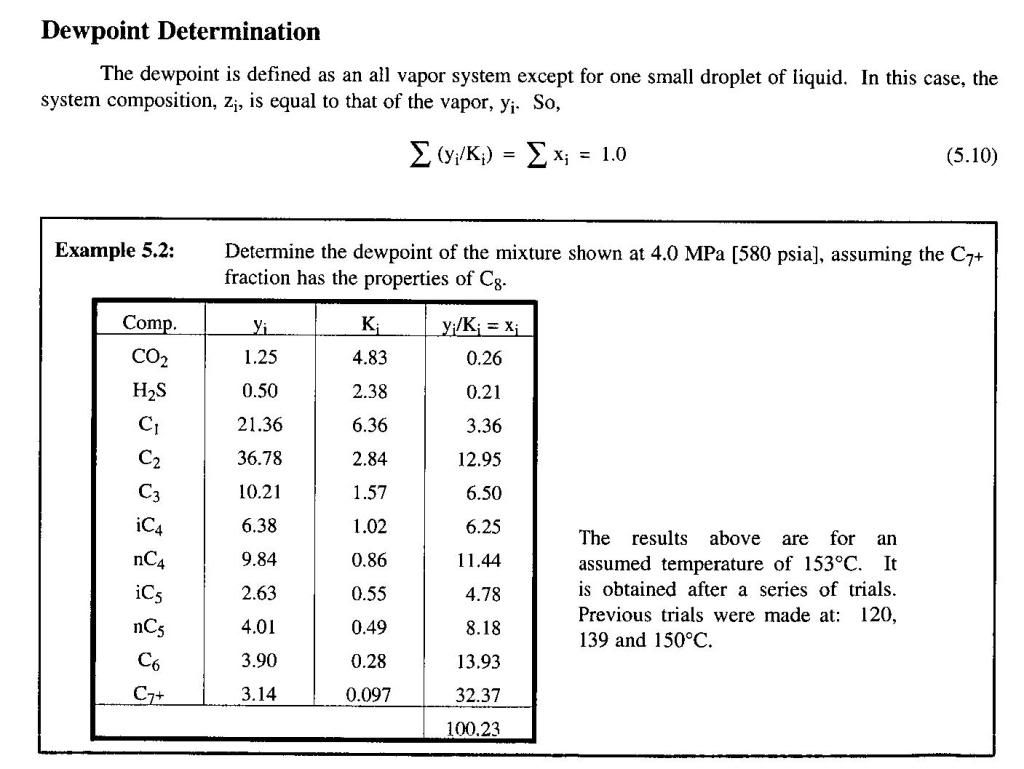
Please repeat this equations to get the result.
Best regards.
LuuQuocDai
luuquocdai@gmail.com
#7

Posted 19 April 2009 - 09:37 PM
QUOTE (luuquocdai @ Apr 19 2009, 04:27 PM) <{POST_SNAPBACK}>

Please repeat this equations to get the result.
Best regards.
LuuQuocDai
luuquocdai@gmail.com
how do i get Ki values using equation of state.
#8

Posted 19 April 2009 - 10:22 PM
dear siddiqsh,
please look up the chapter dealing with non-ideal VLE in any standard chemical engineering thermodynamics textbook, like Van Ness et. al. for example.
i think you'd learn alot more if you actually went to the library and do some looking up before coming here asking to be spoon-fed.
clarence
please look up the chapter dealing with non-ideal VLE in any standard chemical engineering thermodynamics textbook, like Van Ness et. al. for example.
i think you'd learn alot more if you actually went to the library and do some looking up before coming here asking to be spoon-fed.
clarence
#9

Posted 19 April 2009 - 11:30 PM
Hi ,
If I'm right you can found all the info in Process heat transfer from KERN and Perry's chemical handbook.
Regards
Breizh
If I'm right you can found all the info in Process heat transfer from KERN and Perry's chemical handbook.
Regards
Breizh
#10

Posted 20 April 2009 - 05:32 AM
You can find the value of Ki in Gas Conditioning and Processing of John Campbell
If you have any question about this problem, please contact or reply as soon as possible.
Best regards.
LuuQuocDai
luuquocdai@gmail.com
If you have any question about this problem, please contact or reply as soon as possible.
Best regards.
LuuQuocDai
luuquocdai@gmail.com
Similar Topics
Separation Technology For Separate / Distillate Hcl From Water MixtureStarted by Guest_dpunnn_* , 11 Sep 2024 |
|

|
||
Prv Fire Case Load Calculation For Mixture.Started by Guest_Halflight_* , 24 Jun 2024 |
|

|
||
How To Find K-Values Of A Hydrocarbon Mixture On Aspen Hysys?Started by Guest_Alexis Azamar_* , 08 Apr 2024 |
|

|
||
Hydrocarbon Dew Point Control UnitStarted by Guest_BabRafiq1_* , 05 Apr 2024 |
|

|
||
How Calculate Amount Of Water For A Water Saturated Hydrocarbon StreamStarted by Guest_AnbIran_* , 04 Oct 2023 |
|

|

 FB
FB








