Chemical and Process Engineering Resources
Fume Control and Scrubbing on Hydrochloric Acid Pickle Lines
Nov 08 2010 01:20 PM | Chris Haslego
New standards for hydrochloric acid emissions are expected to be proposed by the EPA soon, and these will affect the design of scrubbers for pickle line fume exhaust systems. This paper discusses hydrochloric acid fume scrubbing, with particular reference to the impact of new regulations on the type and design of equipment required.
At this time, the new standards for hydrochloric acid scrubber efficiency are not known, but we can be sure that they will be more rigorous than present requirements. At present, most fume scrubbers are designed for efficiencies of 90-95% - new requirements are expected to be in the 98-99% range. What does this mean for the pickle line operator?
Why Scrubbers are Needed
The liquid hydrochloric acid used in pickling is actually a solution of hydrogen chloride gas in water. Raw acid is generally purchased as ‘20°Baumé’, which means it has a specific gravity of 1.16 and contains about 32% hydrogen chloride (HCl) and 68% water. For pickling purposes, this acid is usually diluted to about 18-22% HCl.
The HCl solution used in pickling emits HCl vapors into the space above the liquid surface. The tendency of the HCl to escape is measured as a vapor pressure - the higher this pressure, the more HCl escapes. Figure 1 shows the vapor pressure curves for HCl at various temperatures as a function of liquid concentration - the vapor pressure increases as temperatures and concentrations increase. These curves are for the vapor pressure over solutions in pure water - the vapor pressures over pickling acid solutions are significantly higher, due to the accumulation of iron chloride in the acid, which increases the solution HCl vapor pressure.
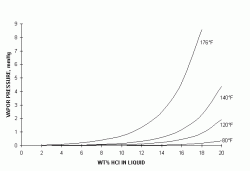 |
| Figure 1: Vapor Pressure of Hydrogen Chloride Over Aqueous Solutions (ref 1,2) |
Figure 1 shows why pickle acids need fume control. The threshold limit value (TLV) for HCl is 5 ppmv, which is equivalent to a vapor pressure of about 0.004 mmHg. This pressure is in equilibrium with a 5% solution at 120°F, 2% at 140°F and less than 1% at 176°F - all concentrations well below what is used in pickling, whether in open or closed tanks. This means that the air above the surface of the acid during pickling is well above the allowable limits, and some form of containment or control is needed.
Besides operator hygiene, control is needed because HCl is extremely corrosive to the building fabric and equipment. Referring again to Figure 1, the 80°F curve shows that there is negligible vapor pressure up to about 5% - this means that HCl is very soluble in cold water, and even low gas concentrations can generate high liquid acid concentrations. Thus, HCl in the air can migrate through the interior fabric of the building, and dissolve in condensation on cold surfaces, producing a strong and corrosive acid in hidden or inaccessible areas.
The ideal fume control would be to pickle in a sealed vessel, isolated from the air - however, the need for openings to insert, guide, access and remove the steel from the acid generally precludes this, so usually the fumes are controlled by creating a draft above the acid surface. This causes air to be drawn in through the openings, thereby preventing escape of acid fumes. However, as the air flows across the acid surface, it sweeps away the HCl vapors, which are then carried out of the tank in the exhaust air. The concentration of HCl in the exhaust gas is a complex function of tank open surface area, air velocity, temperature and acid composition, and can vary from 50 ppmv in well-controlled open tank systems with lateral exhaust, to as much as 2000 ppmv in continuous pickle lines with tight lids and 6000 ppmv in tower picklers. These concentrations are far too high for discharge to the atmosphere, even without considering the effect of such emissions on the building roof and nearby structures.

 FB
FB

0 Comments