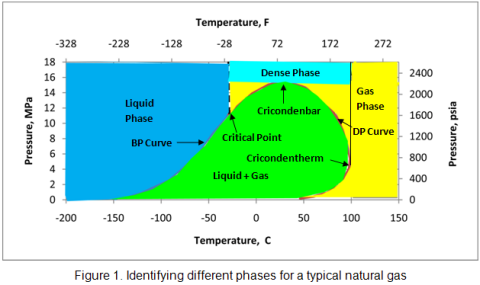
Dear all,
I just got a project for a LNG vaporizer on an LNG carrier. The ship runs with a duel fuel engine, means with high pressure gas instead of diesel. My problem is that my supplier of the vaporizer is not 100% that sure liquid converts into gas due to the high pressure.
The prozess is as follow.
- Main tank with LNG
- a high pressure pump increase the pressure to 320bar into the vaporizer.
- the input temperature of the LNG into the vaporizer is -140C degree
- the output temperature of the vaporizer +45C degree and 320bar
Does someone know if the LNG is still in liquid form at the outlet of the vaporizer or if it changes into vapor?
Thanks in advance
Joerg

 FB
FB