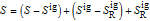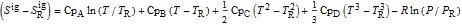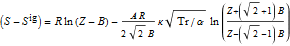Hi,
I'm trying to calculate the thermodynamic properties of a pure ethylene stream as it goes through a compressor. I know the inlet conditions of S,H,T,P and i know the outlet pressure of the stream and the adiabatic efficiency of the compressor is 75%.
However, i'm not not sure how i can use the peng robinson EOS to find out the temperature of the exiting stream for 100% adiabatic efficiency. I know i can equate the entropy change function to zero. But the function contains Z. What is the way that this problem is normally solved?
Thank you!
 is in kJ/mol and
is in kJ/mol and 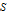 is in kJ/[mol K]; the superscript
is in kJ/[mol K]; the superscript 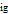 represents an ideal gas, the subscript
represents an ideal gas, the subscript 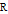 refers to the reference state, and
refers to the reference state, and 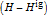 and
and 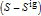 are the enthalpy and entropy departure functions for a real gas calculated from the Peng–Robinson EOS, while
are the enthalpy and entropy departure functions for a real gas calculated from the Peng–Robinson EOS, while 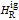 and
and 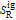 are the ideal gas enthalpy and entropy at the reference state.
are the ideal gas enthalpy and entropy at the reference state.
 FB
FB