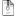I did research on topic of separation of ABE products and found that one of the method used is 3 distillation Columns. In the first one acteone ethanol and water are sent to the top stream, and the butanol sent to the bottom stream. The top stream is sent to a distillation column to separate ethanol and acetone. The butanol stream continues to another distillation column to be separated from water.
I attached a diagram of the process.
I was wondering why not separate the components based on volatility?
First acetone, then ethanol and lastly Butanol.
One reason I can think not to do it is there might be more azetrope mixtures: ethanol-water,butanol-water.
------>harder separation---->more expenisve unit operation to perform the separation----->increase in capital cost.
I would love to hear your inputs on the subject.

 FB
FB