- Cheresources.com Community
- → Highest Reputation Content
Highest Reputation Content
#5255 Uploading Ms Excel Spreadsheets
 Posted by
Chris Haslego
on 18 March 2006 - 05:08 PM
Posted by
Chris Haslego
on 18 March 2006 - 05:08 PM
#96123 Revision Calculos Tanque Api 650, Edicion 12, Marzo 2013
![]() Posted by
Rafael David
on 17 June 2015 - 05:40 AM
Posted by
Rafael David
on 17 June 2015 - 05:40 AM
Señor TALVI y demás miembros del Blog adjunto memorias de cálculos en Excel para su revisión técnica, ingeniería y conceptos que ustedes consideren que se deben tener en cuenta en este diseño.
La idea es que revisen estas memorias y la complementen con sus conocimientos y experiencia.
En este proyecto que estoy desarrollando se van a construir doce tanques así: Cuatro (4) de acero al carbono en SA-36, uno (1) en Acero inoxidable tipo 316 y seis (6) en PRFV o fibra de vidrio, con capacidad cada uno de 42.5 ton. para almacenar productos para químicos para tratamientos de aguas.
En las memorias están los datos del este producto del tanque N° 1 de acero al carbono. Mi objetivo es que este software en Excel le sirva a todos los miembros de la comunidad.
a la espera de su valioso aporte.
Saludos
Attached Files
-
 MEMORIA -TANQUE N 1° LA2326C.xls 3.61MB
643 downloads
MEMORIA -TANQUE N 1° LA2326C.xls 3.61MB
643 downloads
#82070 How About An "in Development" Section In Downloads?
 Posted by
ankur2061
on 19 January 2014 - 01:14 PM
Posted by
ankur2061
on 19 January 2014 - 01:14 PM
Sharing your work (calculations / excel workbooks) can be done only with an open heart and a mind free of suspicion.
It is outright hilarious when people claim that the calculation they have created in the form of an excel workbook is their original work. Most of the engineering calculations that we do today have their roots in the physical laws, principles, formuals, and equations developed by pioneers and researchers in the field of engineering and science long before many of us had even understood what engineering calculations are.
People who are developing calculations / excel workbooks are only organizing and representing the work in a proper sequence and in a manner which is easy to understand and present. Yes, it takes time and effort to do this, and those who have undertaken this task need to be appreciated.
I have never endorsed spoon feeding to young engineers because it allows them to think that there is an easy way out for everything. Many of the blog entries in my blog are related to engineering calculations where I have exhorted the engineers to prepare excel workbooks based on the calculation steps mentioned. For each and everyone of these blog entries related to chemical engineering calculations, I have developed an excel calculation workbook, which I have not shared for the very same aforementioned reason.
However, I do want to make a public pledge today. When my eyesight starts betraying me and my hands are no longer able to type on the keyboard of a computer, I would like to donate my entire set of chemical engineering calculation workbooks to "Cheresources" for Chris Haslego to use in a manner he deems fit for the good of the chemical engineering community.
Regards,
Ankur
#93253 Condensate Pot Equalisation Line
 Posted by
Art Montemayor
on 27 February 2015 - 01:31 PM
Posted by
Art Montemayor
on 27 February 2015 - 01:31 PM
I’ve lost count of how many equalization line “problems” I’ve resolved in the field in the past 55 years. It must be around 10 to 20. In almost every one of these so-called “problems” the cause has always turned out to be a cheap, quick, and last-minute process engineering or piping designer “solution” to a basic process design screw-up. I’ve resolved the problem as shown in the attached revised sketch.
The typical situation is where a process engineer turns over the process design to a mechanical engineering team composed of usually mechanical engineers and piping designers (who are not engineers). The basic process design package should contain all the process information critical to mechanically designing and fabricating a reliable and controllable piece of equipment. This involves the correct sizing of attached piping, nozzles, vents, safety valves, drains, and EQUALIZATION lines. Their locations are also required to be specified. All this information and its communication should be carefully organized and scheduled according to project needs. What usually happens in the case where a heat exchanger - especially a steam-heated one - is concerned is that the process engineer turns over his deliverables to a heat transfer engineer who produces the heat exchanger design for fabrication. The need for an equalization line is left to a piping designer’s decision or criteria. In this process, there is inevitably an oversight in failing to point out the need to control the production, flow and control of steam condensate produced in the exchanger. The fabrication drawings for the exchanger go out approved without any thought as to how the condensate flow will be gravity drain-controlled and this become apparent when the exchanger is delivered. The solution to ensuring that the condensate flow is allowed to drain under gravity to the respective condensate drum: quickly connect the condensate drum to the steam supply line. This is quick, cheap, and presumably vents the condensate to a pressure “equal” to that where it originates, and it eliminates the need to do a re-work on the exchanger bonnet (possibly requiring an ASME stamp, expensive and time consuming). WRONG! The steam supply pressure is NOT equal to the condensate origin pressure - it is higher by the exchanger’s pressure drop. The term “equalization” means exactly that: the pressure in the drum should equal the pressure at the condensate’s origin. This allows for free, un-interrupted gravity flow. The true and correct origin pressure unfortunately exists in the exchanger’s bonnet last tube pass. This fact should have been specified by the process and exchanger engineers prior to exchanger fabrication. An inexperienced or flawed engineering design has caused a more expensive, correct modification. In today’s engineering world, this type of flawed, embarrassing design is becoming more repetitive than engineering houses would like to admit.
I have preached this reality countless times on our forums and have probably become an un-welcomed bore. I promise this will be the last time. Samroo’s recommendation is the correct answer.
Attached Files
-
 Condensate Pot Equalization Line.xlsx 14.09KB
501 downloads
Condensate Pot Equalization Line.xlsx 14.09KB
501 downloads
#122007 I Need 2020 Cepci
 Posted by
breizh
on 02 November 2020 - 07:17 AM
Posted by
breizh
on 02 November 2020 - 07:17 AM
Hi,
The last one on hands is from august 2020 .
Sorry I don't have anymore a subscription to CHE .
Good luck
Breizh
#88971 Chemical Engineering Plant Cost Index (Cepci)
 Posted by
breizh
on 25 August 2014 - 05:59 PM
Posted by
breizh
on 25 August 2014 - 05:59 PM
Hi,
Year 2013 : 567.3
Final April 2014 : 573.6
Preliminary May 2014 : 574.4
Breizh
#60483 How About An "in Development" Section In Downloads?
 Posted by
Chris Haslego
on 24 May 2012 - 08:56 AM
Posted by
Chris Haslego
on 24 May 2012 - 08:56 AM
However, some people may be working on similar projects and may find these helpful. My hope would be to release them in the Downloads section (clearly marked as "Development Items") and to see if the community can work together to turn these into polished projects.
I thought I'd start by collecting initial feedback on the idea. Let me know what you think. These titles would only be visible to registered members of the community.
Thanks,
#133808 Liquid Liquid Separator Sizing
 Posted by
breizh
on 03 April 2025 - 04:16 AM
Posted by
breizh
on 03 April 2025 - 04:16 AM
Hi,
Consider this resource from Bobby. He was a member of this community.
http://www.bobby-str...I_Decanter.aspx
Do you have access to real solutions? if yes perform test to get data about the decantation time. Several parameters to consider, temperature (viscosity), ratio aqueous phase / organic phase (could be modified by recirculation of one phase), coalescers to reduce the size of the decanter. Based on 8 years' experience designing and operating Mixer settlers for rare earths purification.
Check your document ( flow rate should be expressed in Kg/h )
Another resource could be Perry's chemical engineering handbook.
Breizh
Attached Files
-
 Liq-liq_Separations_HP_June09.pdf 1018.36KB
52 downloads
Liq-liq_Separations_HP_June09.pdf 1018.36KB
52 downloads
-
 liquid -liquid coalesceur.pdf 940.25KB
40 downloads
liquid -liquid coalesceur.pdf 940.25KB
40 downloads
-
 Liquid_Liquid_Separation_Technology.pdf 3.14MB
49 downloads
Liquid_Liquid_Separation_Technology.pdf 3.14MB
49 downloads
#132207 Spillback In Centrifugal Compressors
 Posted by
Art Montemayor
on 14 June 2024 - 06:02 PM
Posted by
Art Montemayor
on 14 June 2024 - 06:02 PM
Venkat:
Attached is a Centrifugal Compressor document I put together to help my grandson who's taking Environmental Engineering in college. I believe it answers your query - plus it furnishes a lot of other basic information on centrifugals.
I installed and operated a lot of gas compressors in my time and I was often reminded of the trade offs that come with the centrifugal's simplicity, cost savings, and smaller space requirements as compared to reciprocating units. Surge and Stonewalling are some of those trade offs. I hope this helps you understand centrifugal features and operations.
Take good care of your compressors, and they'll take care of you.
I always preferred reciprocating over centrifugal machines when given a choice. Power efficiency, versatility, ease of maintenance, and flow controls were some of the good features.
 Centrifugal compressor.docx 1.64MB
72 downloads
Centrifugal compressor.docx 1.64MB
72 downloads
#126492 Closed Drain With Sump
 Posted by
Art Montemayor
on 29 May 2022 - 09:55 AM
Posted by
Art Montemayor
on 29 May 2022 - 09:55 AM
Ghasem:
This is a very poorly written request for help.
- We don't know how this can be within the Relief Devices Forum. It is not a relief device.
- Is the Closed Drain located on shore? Off shore?
- Are you a student? Your request is very amateurish. You don't give specific, detailed, and illustrated information on what your query is.
- Is this an academic assignment? Or is it real life?
- Why the need for heat or vaporization? Be specific.
- What kind of process environment is this closed drain meant to service?
- Furnish a detailed P&ID of the process, complete with a written description.
- What do you mean by "Basis for selection"? Be detailed and specific.
- A closed drain is there to protect personnel and the environment. This is a very serious and important item and requires a lot of specific, accurate, and detailed information if our members are to give suggestions or recommendations.
You are requesting specific and detailed recommendations. The same type of information is required by the Forum. The quality and detail of the response is directly proportional to the quality of the request.
And don't fail to fill in the requested information in your personal information form.
Await your response.
#125827 Tsa Bed Regeneration Heat Energy Calculation
 Posted by
Art Montemayor
on 17 March 2022 - 05:03 PM
Posted by
Art Montemayor
on 17 March 2022 - 05:03 PM
Bitan729:
I presume you are dealing with an adsorption unit operation that is dependent on temperature bed regeneration – commonly called a “TSA”. I’ve designed and built this type of adsorption unit and have dealt with the subject at length. I don’t understand why you’ve posted this thread in the Process Simulation section. What you ask has little or nothing to do with simulating adsorption.
First and foremost, your needs to define the sorption and desorption abilities of your adsorbents should be addressed to your adsorbent supplier. Today, there are many – in different countries, and with different degrees of performance and credibility.
I don’t know whose process and cold box you are using to produce both gaseous and liquid Nitrogen, but I note that your feed air supply is at a rather low 110 psig. What this indicates is that your water load is much larger than what I’m used to in the past: 1,000 to 2,000 psig. Your adsorbers are going to be larger beds.
I’ve designed and operated my adsorbers for superficial velocities of 20-50 ft/min and I’ve heated my regenerated beds to 400 – 500 ºF, far in excess of your 375 ºF gas flow. I started with 350 ºF gas flow temperatures as regen gas and as I got more experienced and obtained more professional advice and knowledge, I wound up with getting the beds to reach the higher 400-500 ºF. This not only produced better operating results, it also increased the adsorbent operating life. At the end of my career in compressed gases, I fully expected and got 3-5 years of operating life from my adsorbents which included ALCOA’s Activated Alumina and Linde’s Molecular Sieves.
Not knowing what you are operating – or how – I can’t offer any more comments. I offer you the attached interesting article on the adsorption operation in compressed gases.
Some recommended adsorbent bed regeneration temperatures for common adsorbents are as follows:
Adsorbent
Regeneration Temperature
Molecular Sieve
450 °F to 600 °F
Activated Alumina
400 °F to 500 °F
Silica Gel
400 °F to 500 °F
The required regeneration gas temperature exiting the heater is a function of the regeneration gas water content, the regeneration pressure and the desired level of dehydration or final product gas water content. An adsorbent supplier should be consulted for specific cases. Note: the regeneration bed temperature is different from the regeneration gas temperature.
 Mol Sieve Dehydration Design and Operation.docx 329.61KB
81 downloads
Mol Sieve Dehydration Design and Operation.docx 329.61KB
81 downloads
#124688 Water In Diesel Due To Condensed Moisture From Air Ingress From Vents
![]() Posted by
PingPong
on 31 October 2021 - 07:58 AM
Posted by
PingPong
on 31 October 2021 - 07:58 AM
I am not an expert on storage matters but I will give you my opinion anyway:
Recently we have found that there are a lot of free water in our diesel system............A root cause analysis has been done by the operation team members and the air ingress through the vents into the system and moisture condensation was considered as a main suspect. (An estimate amount of 30 to 50 liter per day of moisture condensation). During the previous 2 weeks we have drained about 20 m3 free water from the system.
20 m3 in 2 weeks would be 1400 liter per day on average, not 50 liter per day.
Lots of water condensation from ambient air in diesel tanks seems highly unlikely to me.
You indicate that the problem is recent, so the ambient air is unlikely to be the problem. Climate change does not occur that fast.
You should however check for any rain water leakage.
For water to condense from air it requires contact with a surface that is colder than its dewpoint.
According to the drawing each diesel tank has an electric heater on TC. What is the setpoint of this TC ? What is the dewpoint of the ambient air?
As the problem is recent: what changed just before the problem started? That's what you should focus on.
If nothing changed inside your unit then something must have changed outside your control.
The most likely suspect is then the supplier of the diesel, or the refinery where the diesel was produced.
Sample the diesel when it is supplied and analyze its water content, not only free but also dissolved and emulsified water.
Also check how long it takes for any water in the diesel to settle completely. Leave it standing in a glass cylinder for weeks if necessary.
Can the centrifugal pump affect the centrifuge performance by making smaller water droplets?
Yes, and what is worse: a centrifugal pump can cause an emulsion if the diesel contains components that act as surfactants. The original pumps where screws, probably to avoid that problem.
And that's where the supplier of the diesel comes in again: maybe there was something changed in the production or handling of the diesel before it reaches your unit, or maybe the diesel now comes from another refinery than until recently.
#124407 Separation Of N-Butane From 1-Butene By Extractive Distillation
![]() Posted by
PingPong
on 22 September 2021 - 08:57 AM
Posted by
PingPong
on 22 September 2021 - 08:57 AM
Maybe you are no using the right thermodynamics in your simulation. You need to use the right activity coefficient model, not simply PR or SRK.
Attached pdf gives a good example. They however do not use DMF but instead acetonitril (ACN), which is also used in the patent under [0019].
First try to reproduce their results with ACN and when successful replace ACN by DMF if that is what you want to use. That may require adjusting other parameters as well such as pressure, temperature, solvent recycle rate, number of trays, et cetera.
 Process Simulation of 1-Butene and N-Butane Separation By Extractive Distillation.pdf 535.12KB
35 downloads
Process Simulation of 1-Butene and N-Butane Separation By Extractive Distillation.pdf 535.12KB
35 downloads
#122921 Useful Oil And Gas Processing Calculations - Part #2
 Posted by
Yasserkassem
on 10 March 2021 - 03:31 AM
Posted by
Yasserkassem
on 10 March 2021 - 03:31 AM
 8- Liquid and gas retention time in separators.docx 18.06KB
40 downloads
8- Liquid and gas retention time in separators.docx 18.06KB
40 downloads
 9- sizing of 2 phase separator with examples.docx 768.24KB
51 downloads
9- sizing of 2 phase separator with examples.docx 768.24KB
51 downloads
 10- design of 3 phase separators with examples.docx 333.24KB
46 downloads
10- design of 3 phase separators with examples.docx 333.24KB
46 downloads
 11- Heat Required for heating crude oil.docx 15.53KB
28 downloads
11- Heat Required for heating crude oil.docx 15.53KB
28 downloads
 12- Salt Content in crude oil.docx 14.53KB
28 downloads
12- Salt Content in crude oil.docx 14.53KB
28 downloads
 13- Gas Orifice and gas rate Calculations.docx 68.77KB
32 downloads
13- Gas Orifice and gas rate Calculations.docx 68.77KB
32 downloads
 14- Liquid Measurements with orifice.docx 149.05KB
28 downloads
14- Liquid Measurements with orifice.docx 149.05KB
28 downloads
 15- Control Valve Sizing for gas and liquids.docx 473.43KB
45 downloads
15- Control Valve Sizing for gas and liquids.docx 473.43KB
45 downloads
Main Site
#117410 Solubility Of Hydrogen Sulfide In Petroleum
![]() Posted by
PingPong
on 18 February 2019 - 04:06 AM
Posted by
PingPong
on 18 February 2019 - 04:06 AM
You don't need to do complicated VLE calculations.
A value for total absolute pressure P is required but normally that is known.
But even if it is not known you can simply assume a value for P to determine H.
For example, using the graph in GPSA EDB, the K-value for H2S in hydrocarbons is:
K = 32 at P = 1 bar & 38 oC
K = 3.2 at P = 10 bar & 38 oC
So at 1 bar Henry constant H = 32 * 1 = 32 bar at 38 oC
and at 10 bar Henry constant H = 3.2 * 10 = 32 bar at 38 oC
It makes no difference what P is because K is inversely proportional to P as long as P is not too high (see graph).
In other words: only at very high pressures H is dependent on P.
#116960 Positive Displacement Pump Curve
 Posted by
Art Montemayor
on 01 January 2019 - 03:46 PM
Posted by
Art Montemayor
on 01 January 2019 - 03:46 PM
Van8888:
Just as Latex and Fallah state: Although you don't tell us the TYPE of positive displacement pump you are using (a piston pump will have essentially no pump curve; it's displacement is constant if both valves work), the pump's volumetric efficiency will increase as the viscosity increases. Rotary type pumps depend on internal clearances (pistons don't) in delivering a specified liquid volume per time. As the viscosity increases, the pump increases its effectiveness due to the thicker fluid. So, a rotary pump's pump curve will be different for different viscosities.
ON A MORE PERSONAL NOTE:
Happy New Year to all our members and a special salute and recognition to our good Breton friend, BREIZH, who has announced his retirement. I hope he persists in joining us here on the Forums and I like his latest personal photo ........ although, like me, he seems to be losing a few hairs.
My personal well wishes go out to all our members and contributors. May this year be one of happiness and prosperity to all.
#115602 Heat Exchangers, Zone Analysis In Excel With Prode
 Posted by
PaoloPemi
on 20 August 2018 - 09:53 AM
Posted by
PaoloPemi
on 20 August 2018 - 09:53 AM
there was a Excel page showing how to calculate properties in different zones I attach a copy, you can examine the VBA code for details,
Prode Properties can export flows or you can calculate from (molar) phase fractions and molar weights of different fractions, see this VBA example
PF = StrLf(Stream)
If (PF > 0.000001) Then ' Liquid present ?
mw = StrLMw(Stream)
Cells(rpt + I, 6) = W * PF * mw / mwm ' Liquid Flow
and so on...
you can calculate mixture properties (density, viscosity, thermal conductivity etc.) in different ways,
Prode Properties exposes methods StrGD, StrLD etc. (there are about 300 methods...)
in this Excel VBA example the procedure calculates vapor properties as
Cells(rpt + I, 14) = StrGMw(Stream)
Cells(rpt + I, 15) = StrGCp(Stream)
Cells(rpt + I, 16) = StrZv(Stream)
Cells(rpt + I, 17) = StrGD(Stream)
Cells(rpt + I, 18) = StrGV(Stream)
Cells(rpt + I, 19) = StrGC(Stream)
of course different variants are possible (many methods available),
you can solve many different Flash Operations with Prode Properties, in this case (heat exchanger simulation) the procedure solves a operation with specified P and H but different alternatives are possible
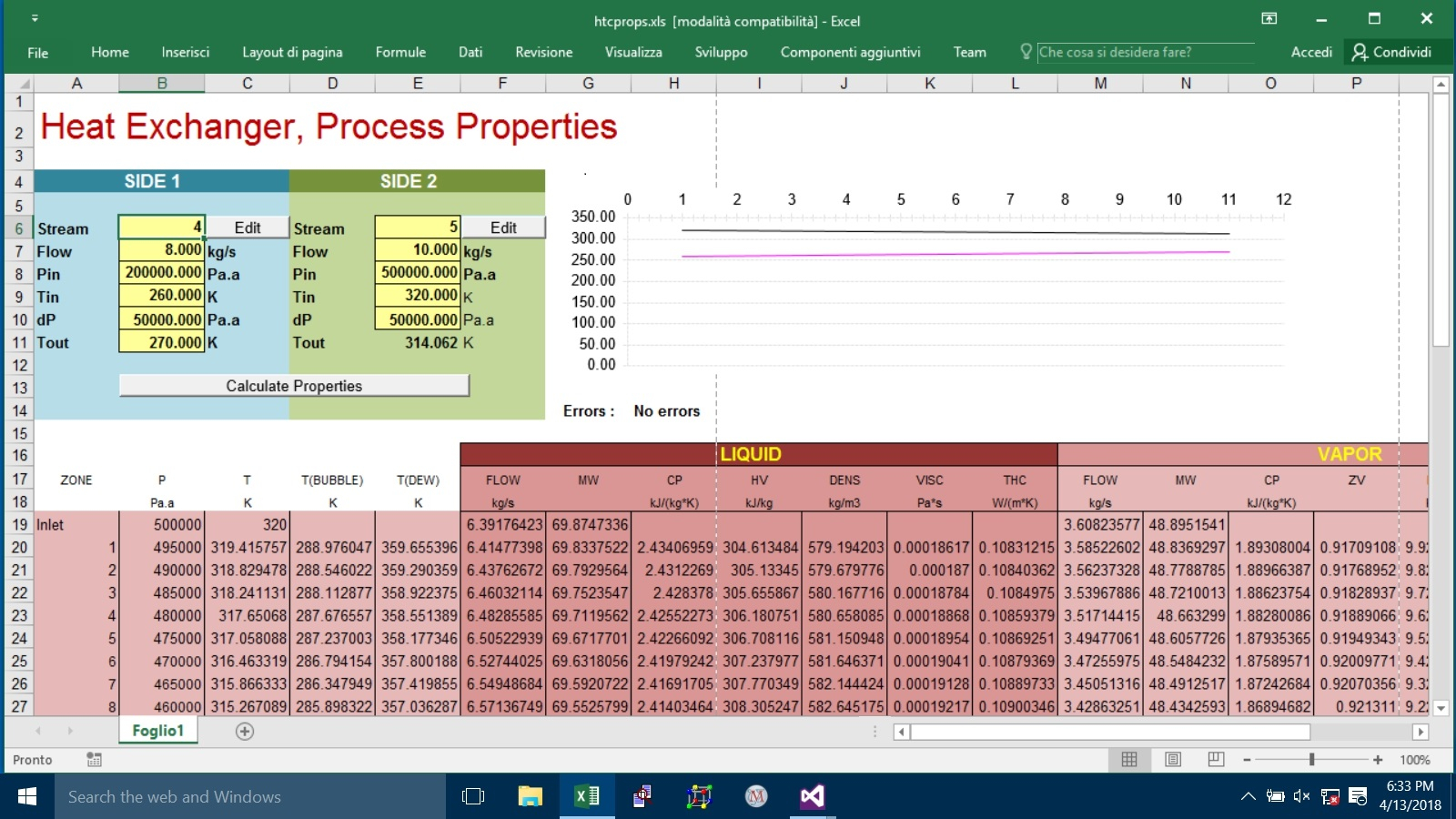
![]() htcprops.xls 176.5KB
54 downloads
htcprops.xls 176.5KB
54 downloads
#111399 Steam To Carbon Ratio
![]() Posted by
Flc
on 03 October 2017 - 03:38 PM
Posted by
Flc
on 03 October 2017 - 03:38 PM
Hi,
In syngas plants (ammonia, hydrogen, methanol, etc.) the concepts are different. Steam to dry gas ratio (usually abbreviated as S/DG) is the ratio between molar flow rate of steam and gas, so it's steam flow rate divided dry gas flow rate. This ratio is use frequently at the inlet of shift reactors (HTS, high temperature shift, and LTS, low temperature shift), in particular to evaluate of the flow fed has enough steam as to prevent iron carbide formation in iron-based HTS catalysts.
Steam to carbon ratio is usually used to evaluate the amount of steam at the inlet of a steam reformer, and is the ratio between the steam and the carbon, in mole basis. Consider that C2H6 counts as 2 carbons, C3H8 as 3, etc. E.g. if you have a stream consisting of a mixture of 3000 mol/h of steam, 500 mol/h of C1, and 200 mol/h of C2, ethane counts twice as much (2 carbon moles), so in this case it would be 3000/(500+200*2) = 3.33. In this same case, the S/DG ratio would be simply (3000/700) = 4.28 (though in practice no HTS reactor operates with such a high value).
Theoretically speaking, in the case of a stream consisting of just steam and methane, S/DG and S/C ratio would be the same. In the case of a stream with only steam, methane, and some nitrogen, the S/DG ratio would differ from the S/C ratio. There are some other ratios used, such as the S/C ratio just for hydrocarbons (so you don't consider inorganic compounds such as CO or CO2) or the S/HCC ratio (you just consider HC C2+).
Hope it was clear enough ![]()
Best regards
#107463 Design Of Distillation Column
 Posted by
Art Montemayor
on 23 January 2017 - 11:57 AM
Posted by
Art Montemayor
on 23 January 2017 - 11:57 AM
Mohammed:
To learn how to process design a distillation column you can do a variety of things:
- You can take a first year chemical engineering Unit Operations course in a university that offers such a study;
- You can purchase and study a book on the subject; some books are:
Douglas, James M., Conceptual Design of Chemical Processes, McGraw-Hill, 1988, pp. 453-457.
Kister, Henry Z., Distillation Design, McGraw-Hill, 1992, pp. 275-282.
Luyben, William L., "Introduction" in Practical Distillation Control (W.L. Luyben, ed.), Van Nostrand Reinhold, 1992, pp. 10-11.
McCabe, W.L., J.C. Smith, P. Harriott, Unit Operations of Chemical Engineering, 5th Edition, McGraw-Hill, 1993, pp. 560-568.
Seader, J.D. and Ernest J. Henley, Separation Process Principles, John Wiley, 1998, pp. 305-312. - You can study the material found in many web sites on the internet. One such site is Dr. Randel Price's (http://facstaff.cbu.edu/rprice/) and a sample of his lecture notes is attached.
 7-Distillation - Principles.docx 41.13KB
143 downloads
7-Distillation - Principles.docx 41.13KB
143 downloads
 8-Distillation II - Modeling.docx 43.81KB
118 downloads
8-Distillation II - Modeling.docx 43.81KB
118 downloads
 9-Distillation III - Operating Equations.docx 51.76KB
115 downloads
9-Distillation III - Operating Equations.docx 51.76KB
115 downloads
 10-Distillation IV - Calculations.docx 65.62KB
147 downloads
10-Distillation IV - Calculations.docx 65.62KB
147 downloads
 11-Distillation V - Ethalpy Balances.docx 39.63KB
104 downloads
11-Distillation V - Ethalpy Balances.docx 39.63KB
104 downloads
 12-Distillation VI - Enthalpy Concentration Methods.docx 63.35KB
96 downloads
12-Distillation VI - Enthalpy Concentration Methods.docx 63.35KB
96 downloads
 13-Distillation VII - Equipment & Column Sizing.docx 56.11KB
143 downloads
13-Distillation VII - Equipment & Column Sizing.docx 56.11KB
143 downloads
 14-Batch Distillation.docx 45.86KB
105 downloads
14-Batch Distillation.docx 45.86KB
105 downloads
#129943 Pressure Relief Valve Set Pressure
 Posted by
fallah
on 17 August 2023 - 02:04 AM
Posted by
fallah
on 17 August 2023 - 02:04 AM
1) Can PSV set pressure be lower than the design pressure of the protected equipment ? Generally in guide books, it is above the design and MAWP of the equipment. However, I was trying to do a more conservative design. For example for a fire case;
Design P of Protected Equipment : XX kg/cm2-g
90% of the Design P : XX*0.9 kg/cm2-g
Set Pressure of the PSV: (XX*0.9) / (1.21) kg/cm2-g (21% for fire scenario)
Relieving pressure of the PSV is (XX*0.9) kg/cm2-g for that case. Can it be done for conservative results ?
2) My second question is: If relieving temperature of the PSV is higher than the pipe spec, could it be a serious problem ? (The distance between the PSV and the equipment is not short.)
Hi,
1) The PSV set pressure shall be equal to or lower than the MAWP of the relevant equipment; hence lower set pressure is more conservative but care should be taken not to be so close to the maximum operating pressure.
2) In general, the relieving temperature shouldn't be higher than the pipe spec; but the relieving temperature of the fire case is exceptional and in such case the relieving temperature could be higher than the pipe spec...
- Cheresources.com Community
- → Highest Reputation Content
- Guidelines / Rules for Posting

 FB
FB

